Improve the prognosis of chemotherapy, the new drug reached the main end point of the phase 3 trial
February 11, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Spectrum Pharmaceuticals announced that its first phase 3 trial of the new drug ROLONTIS (eflapegrastim) has reached its primary endpoint, namely the non-inferiority of the duration of treatment of neutropenia compared to the control drug. The phase 3 trial, ADVANCE, examined the safety and efficacy of ROLONTIS in 406 patients with early-stage breast cancer who were neutropenic due to chemotherapy.

Neutrophils are white blood cells and are the main means of fighting infection. Most chemotherapy-induced cases of neutropenia occur during the first cycle of drug treatment, leading to delays in further dosing, reduction in chemotherapy dose, or early termination of chemotherapy in 10% to 20% of patients. Approximately 20% of patients with severe neutropenia develop severe bacterial infections. More than 60,000 patients are hospitalized each year for neutropenic fever, which often leads to serious infections in patients with neutropenia. The mortality rate of these patients is between 9% and 18%.
Rolontis (eflapegrastim) is Spectrum's first biopharmaceutical. It is a long-acting granulocyte colony-stimulating factor (G-CSF) that stimulates its proliferation process by binding to G-CSF receptors expressed on granulocyte progenitors, ultimately producing functional activation in the bone marrow. Neutrophils.
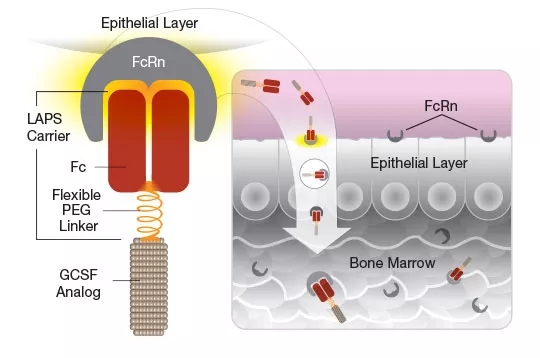
â–²Rolontis drug mechanism of action (Source:Spectrum)
The Phase 3 ADVANCE trial was a multicenter, randomized, active, controlled trial of 406 patients with early-stage breast cancer who received docetaxel and cyclophosphamide chemotherapy every 21 days. In the trial, patients were randomized to receive ROLONTIS or control medication at a ratio of 1:1. Based on a central laboratory assessment of the absolute neutrophil count (ANC) for the 21-day cycle, the primary endpoint of the trial was the duration of severe neutropenia in a single chemotherapy cycle (ANC < 0.5 × 109 / L ).
“The ADVANCE trial confirms the effectiveness and safety of ROLONTIS in Phase 2 trials,†said Lee S., Professor of Hematology Oncology at the University of Tennessee Health Science Center and Director of the UT/West Cancer Center. Dr. Schwartzberg said: "If approved, this drug will be one of the most popular methods of choice for adjuvant treatment of cancer patients undergoing myelosuppressive cytotoxic chemotherapy."

â–² Mr. Joe Turgeon, President and CEO of Spectrum (Source: Spectrum)
“As we continue to drive the company forward, the positive top-line data for this phase III study is an important milestone for Spectrum,†said Spectrum President and CEO Joe Turgeon: “In addition, for this new drug The second phase 3 trial is also nearing completion, which will allow us to submit a Biologics Licensing Application (BLA) in the fourth quarter of 2018."
We look forward to the early launch of this new drug for the benefit of patients with neutropenia.
Reference materials:
[1] Spectrum Pharmaceuticals Announces ROLONTIS (eflapegrastim) Met the Primary Endpoint in the Phase III ADVANCE Study
[2] Spectrum Pharmaceuticals official website
Body Scrub,Coffee Body Scrub,Salt Scrub,Sugar Scrub For Body
Guangzhou Lingxue Cosmetics Co., Ltd , https://www.gzlxgj188.com