Microfluidic chip technology integrates basic operation units such as sample preparation, reaction, separation and detection in biological, chemical and medical analysis processes onto a micrometer-scale chip, and automatically completes the analysis process, enabling micro-processing from sample processing to detection. It is capable of carrying the functions of traditional biological laboratories and chemical laboratories, has strong development vitality, and has a good application prospect in the field of real-time inspection (POCT).
In vitro diagnosis (IVD) is one of the fastest growing areas in the biomedical industry. At present, the main IVD diagnostic techniques include molecular diagnosis, immunodiagnosis, and biochemical diagnosis. Among them, molecular diagnostic technology has become the "gold standard" for clinical diagnosis because of its high sensitivity, strong specificity and short window period. According to the Ministry of Industry and Information Technology's "Blue Book: 2015 Domestic In Vitro Diagnostic Industry Status", China's in vitro diagnostic market will reach 72.3 billion yuan in 2019, of which gene chip and molecular diagnosis are the two areas with the most development potential.
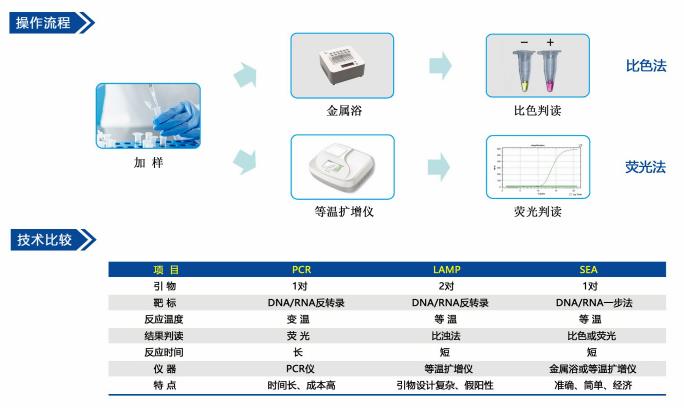
The combination of the advantages of molecular diagnostic precision detection and the integration of microfluidic reaction is the mainstream trend of IVD development in the future, and it has already shown strong momentum and broad development prospects. PCR-based molecular diagnosis is to provide information and evidence for the diagnosis and treatment of diseases by primer-mediated specific amplification of the target gene to detect the presence or absence of the target gene. Its main application scenarios include early diagnosis of diseases, classification of tumors, diagnosis of genetic diseases, prenatal screening, etc. Bohui Innovation has a fully automated, fully integrated microfluidic-based molecular diagnostic platform that subverts traditional The nucleic acid detection process has become a leading company in the industry. In recent years, isothermal nucleic acid amplification technology has sprung up, and the more mature LAMP amplification technology has gradually gained industry recognition. Boao Biotech has developed a LAMP-based amplification instrument that uses microfluidic control, which also opens the door to the combination of isothermal nucleic acid detection and microfluidic control.
>>>>>> Existing microfluidic nucleic acid amplification platforms include isothermal amplification and variable temperature amplification:
1. Variable temperature amplification technology (PCR): It is widely used and mature in technology. However, its amplification reaction requires repeated temperature rise and fall, which increases the complexity of instrument chip design. Therefore, it is more suitable for use in professional laboratories. Microfluidic amplification technology platform.
2, isothermal amplification technology (LAMP): the required primer design is more, easy to non-specific expansion caused by false positive, easy aerosol pollution.
>>>>>>SEA technology
In response to the microfluidic requirements and the shortcomings of the prior art, the research team developed a variant exchange-mediated chain exchange amplification (SEA) with independent intellectual property rights. The SEA method is a denatured bubble-mediated isothermal nucleic acid amplification technique based on DNA "breathing". As with PCR technology, SEA technology only needs to design two upstream and downstream primers to exponentially expand the target nucleic acid region. The difference from PCR technology is that PCR double-strands DNA is opened by high temperature, and the primers are extended and amplified by single-stranded formation after opening (temperature-changing, requiring a temperature-increasing instrument), while the primer of SEA technology is through invading denatured bubbles. The structure, under the action of the polymerase, extends and replaces the original complementary strand to form an exponential amplification (isothermal, without the need for a temperature rise and fall instrument). It is characterized by a short target sequence, which can be applied to detect highly mutated pathogens and pathogenic microorganisms; the reaction is an isothermal reaction, independent of complex temperature rise and fall instruments; the primer design is simple and the technical requirements of the operator are low.
>>>>>>SEA technical advantage
SEA has specially carried out innovative research and development for the whole process of rapid inspection and developed a new generation of nucleic acid rapid inspection technology.
1. Target: One-step detection of RNA is realized, and DNA can also be measured.
2, detection: isothermal detection is simple and convenient, about 20min can quickly determine the results, and is not easy to pollute.
3. Results: The test results can be interpreted by the instrument or by the naked eye.
4. Cost: The SEA primer is simple in design, requires a small amount of enzymes, has low cost, and is simple in process.
5, application: better applicability and scalability, compatible with existing equipment, suitable for combination with microfluidics.
In summary, SEA technology, as a new generation of nucleic acid rapid inspection platform, is accurate, fast, simple, and not easy to pollute. It is easier to combine with other technologies and microfluidic control to create the best solution for microfluidic products. Welcome to call to discuss exchanges and seek cooperation and development. !
Qingdao Naide Biotechnology Co., Ltd.
Contact: Mr. Ma
contact number
mailbox:
Specifications
14.Battery charge: by USB charge or wireless charge, take 2 hours
Advantage
How it works
1. Scan mode: array probe, 4D scan;
2. Supporting system: Apple iOS;
3. Measuring range: 10ml ~ 2000ml;
4. Automatic measurement inaccuracies: <5%;
5. Scan and process<2 seconds;
6. Scanned image display frame: 10 frames / seconds;
7. Case Storage: by tablet or mobile phone;
8. Printing: external wireless thermal printer;
9. Battery: Built-in battery, charge one time can work on more than 200 patients;
10. Power: Wireless charge or by USB cable charge;
11. Dimension: 180mm (length) × 60mm (width) × 60mm (thickness);
12. Weight: 420g.
13.Battery working time: 3 hours(according to whether keep scan)
- Only one probe, convenient for carry
- Wireless, convenient for operation
- 4D array scan, very clear image
- High accurate (5% inaccuracies)
- High speed on scan and process (2s)
- Without distortion of mechanical scanning
- More advanced algorithm, more powerful bladder wall recognition technology(High recognition
rate for the bladder wall with air), more accurate contour technology, higher accuracy of
the probe makes scanning results more accurate.
- New algorithm, the measurement results are not subject to the bladder shape and size.
No need to select Gender, Age group and no need to consider special cases such as the effect
of hysterectomy on the measurement results. Good compatibility for different bladders, no
Ubstantial error when measuring small volume or special shape bladders, easier to operate
and use.
- High precision rotary encoder, precise positioning, never need to calibration.
- Waterproof probe
The Sonostar wireless probe is a mini ultrasound scanner without a screen. We minimize the components of a traditional ultrasound into a small circuit board built into the probe, and showing image in smart phone/tablet through Wifi transferring. image can both show in screen and tablet.Image transferring through internal wifi from probe, no need external Wifi signal.
Bladder Ultrasound Scanner,Bladder Ultrasound For Old Folk,Bladder Ultrasound Machine With 4D,Bladder Scan With Ultrasound
Guangzhou Sonostar Technologies Co., Limited , https://www.sonoeye.com