Fluorouracil (Fu) is the most commonly used uracil antimetabolite and plays an important role in the treatment of oncology. However, due to drug resistance, its efficacy in the treatment of colorectal cancer is greatly limited. An article published in the international journal Nature Communications in March 2019 introduced the key to the sensitivity of colorectal cancer to FU drugs. The main unit was the Department of Oncology, the First Affiliated Hospital of the Army Military Medical University (Chongqing Southwest Hospital). The authors include Professor Li Jianjun and Professor Liang Houjie.
According to the US epidemic of colorectal cancer statistics, nearly 20 years of morbidity and mortality in the United States continued to decline and, according to data released by China's National Cancer Center, China's incidence and mortality of colorectal cancer has continued to rise. Since the early 1990s, the use of a uracil-like drug, fluorouracil (Fu), alone or in combination, has been the primary chemotherapy for patients with colorectal cancer. Although widely used clinically, its drug resistance severely limits the efficacy. Therefore, there is an urgent need for new strategies to resist drug resistance, and understanding the mechanism by which cancer cells develop resistance to Fu is an important step in preventing or overcoming drug resistance.
Macroautophagy is a cellular catabolic process. Autophagy has the potential to supply energy to all nearby metabolic pathways, providing enormous metabolic plasticity to cells. Under the challenge of chemotherapy, radiotherapy and targeted drugs, autophagy can improve cell survival and therapeutic resistance. Metabolic reprogramming and abnormal activity of metabolic enzymes are considered to be one of the characteristics of malignant tumors. Department of Oncology, Chongqing Southwest Hospital, Army Military Medical University is committed to tumor chemotherapy resistance research. They have previously found that lipid metabolism related gene ABHD5 plays an important role in tumor suppression in colorectal cancer.
ABHD5 expression was significantly reduced in human colorectal cancer patients. In 2016, they published in Autophagy that ABHD5 plays a key role in maintaining chromosomal stability and protecting genomic integrity by regulating autophagy. These findings prompted researchers to explore the potential role of ABHD5 in regulating chemotherapy response to colorectal cancer.
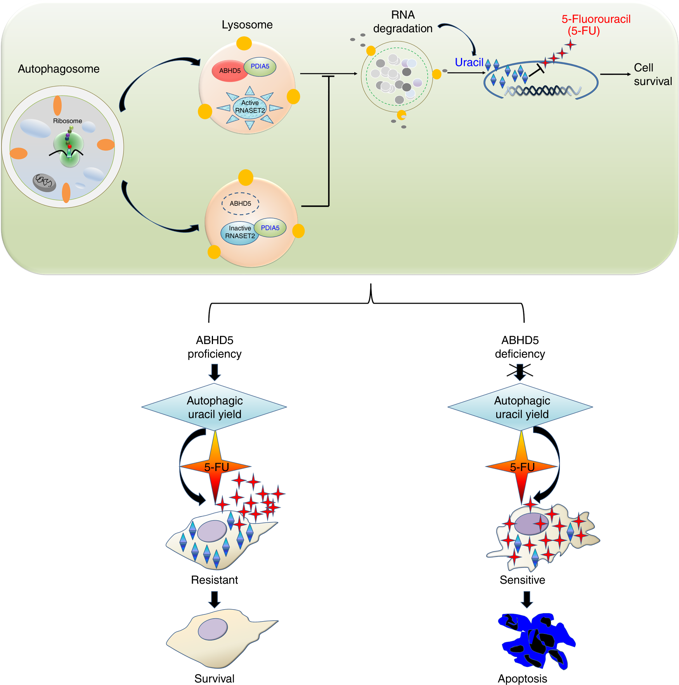
This report reports that although ABHD5 plays a tumor suppressive role in the development of colorectal cancer, it unexpectedly weakens the sensitivity of colorectal cancer cells to FU by promoting RNASET2-mediated autophagy uracil production. This study suggests an important insight into the prognosis of FU-assisted chemotherapy based on ABHD5 status.
They adopted herein for human use Cyagen Biosciences Inc (Cyagen biological) provided colorectal cancer cell lines (of SW480 etc.) first tested the difference between the control group of mice intraperitoneal xenograft injected into the knockout ABHD5. Surprisingly, xenografts from ABHD5 knockout SW480 cells showed a significant increase in sensitivity to FU, showing significant apoptosis (Figure 1).
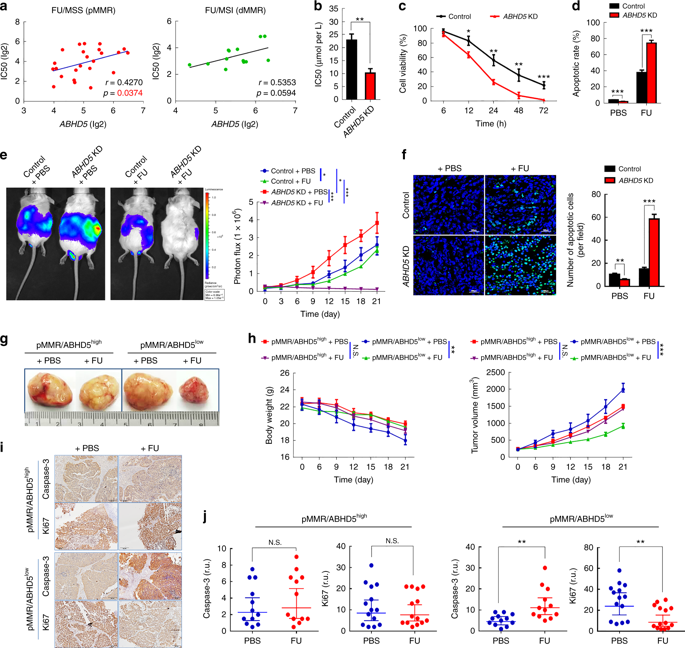
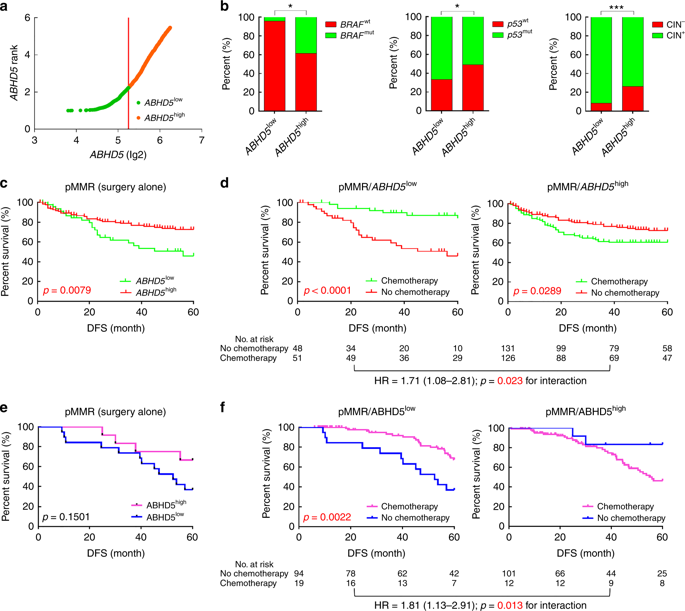
Interestingly, colorectal cancer cell HCT116, which is derived from DNA mismatch repair defects (dMMR), has a relatively small effect on the sensitivity of FU after controlling the ABHD5 variable. PDX mice with normal MMR expression (pMMR) but low ABHD5 expression (pMMR/ABHD5 low ) significantly benefited from FU treatment when the subjects were switched to tumor-specific patient-derived xenograft (PDX) models. Next, pMMR/ABHD5 high mice showed resistance to FU and did not benefit from drug treatment.
ABHD5 high colorectal cancer cannot benefit from FU-based chemotherapy
It has been reported that the prognosis of dMMR-deficient colorectal cancer is improved, but the response to FU-based chemotherapy is poor. About 80% of pMMR patients routinely receive adjuvant chemotherapy based on FU, and even in similar disease stages, patients respond to chemotherapy inconsistently, which brings certain treatment uncertainty.
Can ABHD5 be used as evidence to predict whether patients with pMMR colorectal cancer can benefit from FU-based adjuvant chemotherapy?
The researchers divided 361 pMMR colorectal cancer patients in the NCBI-GEO database into ABHD5 high (ABHD5 high ) and low expression (ABHD5 low ) subgroups, and assessed the relationship between ABHD5 height and prognosis and FU-based adjuvant chemotherapy. In general, the prognosis is better than ABHD5 high subgroup ABHD5 low subgroups, the most notable is that, compared with patients receiving only surgery, patients receiving FU in adjuvant chemotherapy, ABHD5 low subgroups from adjuvant chemotherapy The benefits were significant, and disease-free survival was significantly longer, while the ABHD5 high subgroup was relatively less fortunate, but still longer than the disease-free survival of surgery alone (Figure 2).
ABHD5 reduces FU uptake by increasing autophagy uracil production
Next, the researchers sought to explore the mechanism by which ABHD5 regulates the response of pMMR colorectal cancer cells to FU. First they compared the intracellular FU concentrations of ABHD5 knockout cells and control SW480 cells. The results of high performance liquid chromatography analysis were striking, and the level of FU in the ABHD5 knockout cells was significantly increased compared with the control cells (Fig. 3).
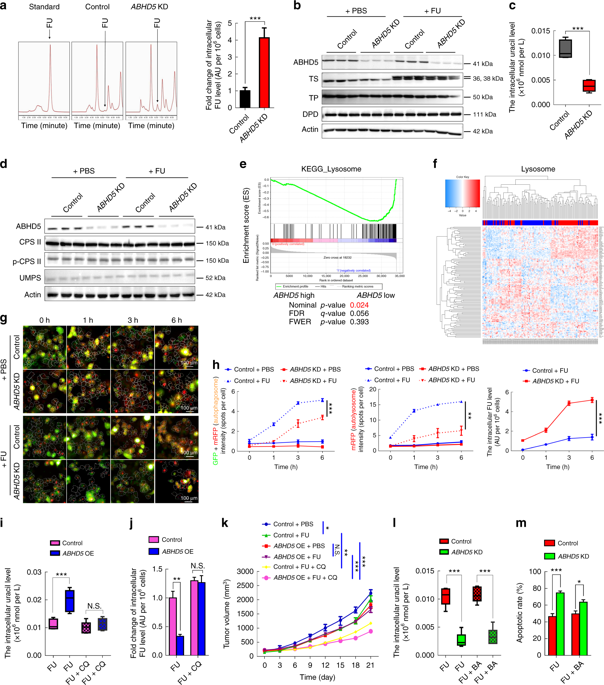
The increase in the level of FU in the ABHD5 gene is due to an increase in drug absorption capacity. However, the metabolic curve shows that the uracil content of these cells is significantly reduced compared with the control group, indicating that ABHD5 deficiency may impair uracil production and promote pMMR junction. Rectal cancer cells use FU as an exogenous source of uracil. In eukaryotic cells, two enzymes (CPS II and UMPS) are responsible for pyrimidine biosynthesis, while ABHD5 knockout cells and control cells have no change in expression levels of these enzymes, suggesting that ABHD5 may be passed through colorectal cancer. A new, independent pathway affects uracil production.
The researchers speculate that lysosomal RNA degradation mediates autophagic uracil production and is attributed to ABHD5-induced increase in uracil in pMMR/ABHD5 high colorectal cancer cells, thereby promoting cancer cell resistance to FU. Subsequently, researchers speculate confirmed a body by targeting autophagy - lysosome fusion tide autophagy inhibitor "chloroquine (Chloroquine)" effectively saved ABHD5 SW480 cells overexpressing FU and uracil concentration. These cell-derived xenografts regained sensitivity to FU. Since the team has previously demonstrated that ABHD5 regulates autophagy by activating BECN1, they suspect that BECN1 is involved in the production of autophagy in this case. However, the results were unexpected. The BECN1 activator only slightly reversed the intracellular uracil of ABHD5 knockout SW480 cells and the response to FU, suggesting that ABHD5 regulates autophagy uracil production mainly through mechanisms other than BECN1.
To find relevant interaction genes, the researchers analyzed the content of uracil-related nucleosides and nucleic acid bases over time by ultra-high liquid chromatography-multiple reaction monitoring mass spectrometry (UHPLC-MRM-MS). During the FU treatment, the cytidine and guanosine content of the control group increased significantly, and the ABHD5 knockout cells showed only a small increase. Various known lysosomal hydrolases degradation of macromolecules (e.g., RNA) is essential, active RNA enzymes to lysosomes may ABHD5 first step RNA degradation due to play a key Promote action, which in turn leads to a decrease in autophagic uracil production (Figure 4).
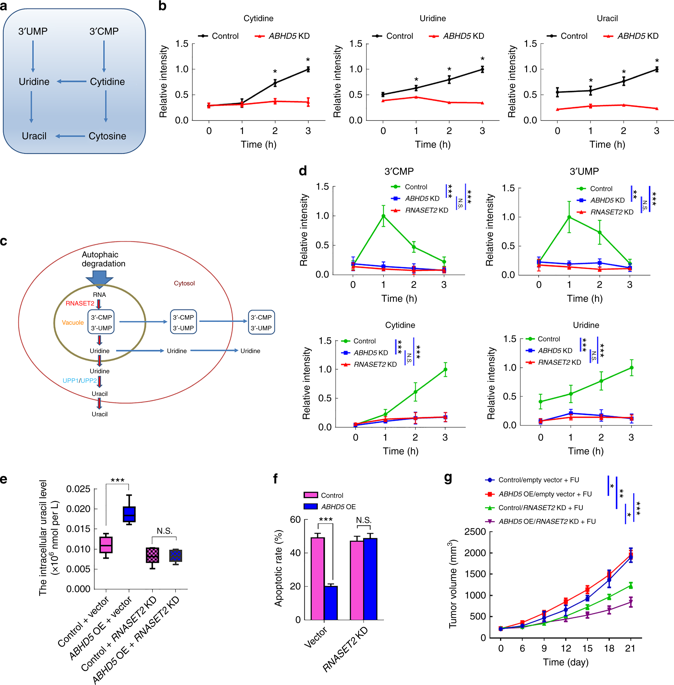
Based on comprehensive data, ABHD5 is localized to lysosomes and shares a common interaction domain in PDIA4 with RNASET2. ABHD5 interact directly with PDIA4 prevent PDIA4 interaction with RNASET2 inactivation RNASET2. Once ABHD5 is defective, PDIA4 interacts with RNASET2, leaving the latter in an inactive state, impairing RNASET2-induced autophagy uracil production.
Absence of ABHD5 promotes colorectal cancer cells to take FU as an exogenous uracil, thereby increasing their sensitivity to FU. In contrast, colorectal cancer cells rich in ABHD5 are inherently resistant to FU due to increased production of autophagic uracil.
This study reveals a new mechanism of chemoresistance in colorectal cancer, and provides new ideas for potential therapeutic strategies to improve the sensitivity of CRC chemotherapy drugs.
Original search: ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield
Cooling Patch,Cooling Gel Patch,Cold Compress Paste,Silicon tape
Surgimed Medical Supplies Co.,Ltd , https://www.surgimedcn.com