Medical Network November 26, November 22, Huahai Pharmaceutical announced that the company received a clinical trial notice issued by the State Food and Drug Administration for valsartan hydrochlorothiazide tablets. Up to now, the company has invested a total of about 36.7 million yuan in research and development.
Basic situation of drugs
The company was notified by the FDA in February 2016 that the company has applied for approval of a new drug for valsartan hydrochlorothiazide tablets filed by the US FDA.
On December 18, 2017, the Drug Evaluation Center of the State Food and Drug Administration issued the “Publication of the Application for Registration of Drugs to Be included in the Priority Review Procedures†(the twenty-fifth batch), and the valsartan hydrochlorothiazide tablets were included in the priority review drug. The registration procedure was announced and publicly announced; recently, the company received a clinical trial notice from the State Food and Drug Administration for the human bioequivalence test of the drug. Up to now, the company has invested a total of about 36.7 million yuan in research and development.
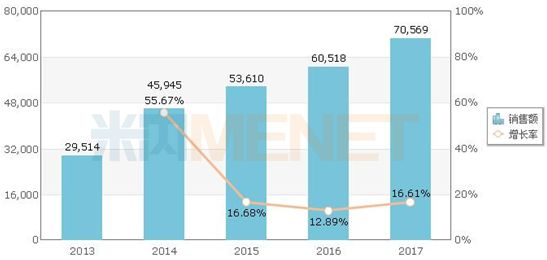
Sales of valsartan hydrochlorothiazide in China's public medical institutions (unit: 10,000 yuan)
Valsartan hydrochlorothiazide tablets are mainly used for the treatment of mild-to-moderate essential hypertension in which a single drug cannot adequately control blood pressure . The valsartan hydrochlorothiazide tablets were developed by NOVARTIS and were marketed in the United States in 1998. In the United States, the main manufacturers of valsartan hydrochlorothiazide tablets are Aurobindo, Mylan, Lupin, etc.; domestic manufacturers mainly include Lunan Beite Pharmaceutical Co., Ltd. and Changzhou Siji Pharmaceutical Co., Ltd.
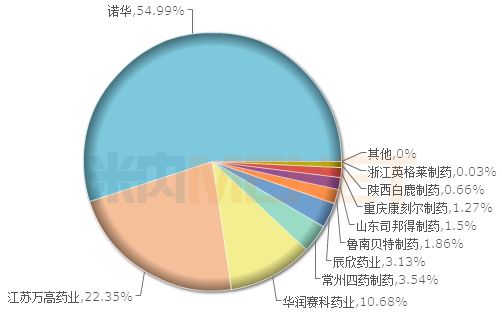
According to the data of the rice, in 2017, the sales of valsartan hydrochlorothiazide in the public hospitals , county-level public hospitals, urban community centers and township health centers (referred to as Chinese public medical institutions) exceeded 700 million yuan, of which the proportion of tablets More than 95%. Among the TOP20 brands, the original research drug company Novartis accounted for 54.99% of the market share.
The TOP20 brand pattern of valsartan hydrochlorothiazide in China's public medical institutions in 2017
Huahai Pharmaceutical said that the company will conduct clinical trials in strict accordance with the notification requirements, and submit clinical trial reports and related documents to the State Food and Drug Administration after the end of clinical trials. At the same time, after completing the BE trial, the products can still be registered through priority review. The program is declared.
   Source: Listed company announcement, Minenet database
GMP ATS Injection, Tetanus Antitoxin, Tetanus Toxoid ,Tetanus Antitoxin Injection, Antitetanus, Refined Tetanus Antitoxinsupplier in China
Tetanus Antitoxin,Tetanus Toxoid,Tetanus Antitoxin Injection,Antitetanus&Refined Tetanus Antitoxin
FOSHAN PHARMA CO., LTD. , https://www.foshanmedicine.com