With the development of scientific research and the advancement of production technology, quantitative analysis of water has been listed as one of the basic items of drug testing, as an important quality indicator for drugs. Under normal circumstances, the abnormal content of water in the drug will seriously affect the quality and efficacy of the drug. In particular, high water content in antibiotics will seriously affect its stability, physical and chemical properties, and the effective period and use effect. Even taking these drugs will seriously endanger the health and safety of the people. Therefore, it is important to check the moisture of the drug and strictly control its limits.
In the past, people used the heating and drying method (ie, the weight loss method) to check the moisture in the medicine. This method is not only cumbersome, time-consuming, and systematically errory, but also the medicines that are unstable in heat cannot be applied to this method, so they cannot meet modern medicines. The need for inspection. In 1935, Karl Fischer discovered a method for determining samples with a water content of from 1 ppm to 100% by titration (Karl Fisher's Moisture Method). The method uses methanol as a medium and Karl Fischer as a titration solution for the determination of sample moisture. The method has the advantages of wide application, simple operation, high accuracy and good repeatability for determining the moisture content, and can ensure the accuracy of the analysis result to the utmost extent. Moreover, the titration time of the method is short, and it takes only 2 to 6 minutes to measure a sample in general, and is especially suitable for a medicine which is easily destroyed by heat. The Pharmacopoeias of China, the United Kingdom, the United States, and Japan all contain Karl Fischer's Moisture Method (capacitometric titration and coulometric titration), and have now become the internationally accepted classical moisture measurement method.
Basic principle
Karl Fischer Moisture Determination is a non-aqueous solution in the non-aqueous solution of iodine and sulfur dioxide in pyridine and methanol solution. The basic principle of titration is Karl Fischer in the electrolytic cell of the instrument. When the reagent reaches equilibrium, the aqueous sample is injected, water participates in the redox reaction of iodine and sulfur dioxide, and in the presence of pyridine and methanol, pyridine hydroiodide and pyridine methyl sulfate are formed, and the consumed iodine is generated at the anode, thereby The redox reaction is continuously carried out until the water is completely exhausted. According to Faraday's law of electrolysis, the iodine generated by electrolysis is proportional to the amount of electricity consumed during electrolysis, and the chemical reaction equation is as follows:
1H2O+I2+SO2+3C5H5N→2C5H5N·HI+C5H5N·SO3 (unstable)
2C5H5N·SO3+CH3OH→C5H5N·HSO4CH3 (stable)
The Karl Fischer reagent contains molecular iodine and is dark brown. When a reagent or sample containing water is added, a solution of pyridine sulfate (C5H5N·HSO4CH3) is formed into a yellow color due to a chemical reaction, and the end point can be judged by visual inspection. That is, from light yellow to orange). Or use "permanent stop titration" to indicate the end of the Kasper reaction, the principle is: insert a double platinum electrode in the reaction solution, add a fixed voltage between the two electrodes, if there is water in the solvent, then There is no electricity pair in the solution, the solution is not conductive; when the reaction reaches the end point, there are I2 and I-electric pairs in the solution. During the electrolysis process, the electrode reaction is as follows:
anode:
2I--2e→I2
cathode:
1 I2+2e→2I-
2 2H++2e→H2
Therefore, the conductivity of the solution suddenly increases, and the current value between the double platinum electrodes provided with the applied voltage suddenly increases, and the stability is determined by setting a threshold value in advance, and the end point of the titration can be judged, and the instrument will The titration is automatically stopped to calculate the water content of the sample by consuming the volume of the Karl Fischer reagent. According to the above principle, the researchers developed the Karl Fischer Moisture Analyzer. In recent years, the more active imported Karl Fischer moisture analyzers in the domestic market are French Reyes, Metrohm, and METTLER TOLEDO, Germany. , Germany SCHOTT,; domestic Karl Fischer moisture analyzer mainly Shanghai Ben Ang and so on. Both the visual method and the permanent stop titration method were included in the Chinese Pharmacopoeia (2005 edition). However, the visual method has a large error and it is troublesome when measuring a colored substance, so the method should not be used. The Karl Fischer Moisture Analyzer is easy to operate, has high sensitivity, good reproducibility, and can be continuously measured and automatically displayed. I recommend that the qualified drug testing department first use the Karl Fischer Moisture Analyzer to measure the moisture in the sample. content.
Two Karl Fischer reagent
Karl Fischer reagents are mainly divided into single component, double component and mixed type, and the bottle Karl Fischer reagent. The Chinese Pharmacopoeia (2005 edition) contains the preparation and calibration methods for the single-component Karl Fischer reagent, which is also the classical volumetric Karl Fischer reagent preparation method. (1) Prepare 110g of iodine (48 hours or more in a sulfuric acid desiccator), place it in a dry conical flask, add 160ml of anhydrous pyridine, pay attention to cooling, shake until all the iodine is dissolved, add anhydrous methanol 300ml, weighed the weight, the conical flask is cooled in the ice bath, under the condition of avoiding the intrusion of moisture in the air, the dry sulfur dioxide is added to the weight increase of 72g, and then anhydrous methanol is added to make 1000ml, tightly plugged, shake , placed in the dark for 24 hours. This liquid should be shaded, sealed, and stored in a cool dry place. The concentration should be calibrated before use. (2) Calibration is directly calibrated with Karl Fischer Moisture Analyzer; or take a dry stoppered glass bottle, accurately weigh about 30mg of distilled water, add 2~5ml of anhydrous methanol, and use this droplet to fix the solution to light yellow. Turn reddish brown, or use the permanent stop titration (Appendix VII A) to indicate the end point; another blank test, calculated as follows:
In the formula WF=A-B, F is the weight (mg) of water equivalent to 1 hour of the test solution; W is the weight (mg) of the distilled water; A is the volume of the test solution consumed by the titration ( M)); B is the volume (ml) of the consumption of the test solution. Since the organic base used in the preparation of the reagent is a odorous odor and a highly toxic pyridine, it is harmful to the health of the tester and seriously pollutes the laboratory environment. In 1984 E. Scholz discovered a new Karl Fischer reagent for the replacement of pyridine with imidazole. This reagent not only replaces the toxic, pungent odor of pyridine, but it also makes the reaction faster and the titration results more accurate. Most manufacturers now produce two types of Karl Fischer's reagents: safe pyridine-free and pyridine-containing reagents, with titers of 3 to 5.
Classification and application range of three Karl Fischer moisture measurement method
Karl Fischer's Moisture Analyzer is applicable to the determination of moisture in a variety of organic and inorganic materials. Due to the differences in the properties of various compounds, it can be divided into direct capacity titration and indirect capacity titration (back titration). However, in the drug testing, direct volumetric titration is often used to determine the moisture content. The typical drugs are as follows:
1 capsule preparation
Azithromycin capsules, cefotaxime capsules, cephalexin capsules, cefadroxil capsules, amoxicillin capsules, etc.;
2 injection powder injection
Cefotaxime sodium for injection, cefazolin sodium for injection, cefoperazone sodium for injection, sulbactam sodium, cefdilam for injection, etc.
3 tablets of amoxicillin dispersible tablets, etc.;
4 raw materials.
Four assay
The Karl Fischer Moisture Analyzer has been used as a major instrument for the measurement of moisture content by the drug testing unit. The ZDJ-400SY full-function titrator (Beijing Pioneer Weifeng) is now used to determine the moisture content.
1 Select solvent
Since this method measures the moisture content of the sample, it is necessary to use a non-aqueous substance as a solvent to dissolve the sample. Under normal circumstances, anhydrous methanol is an ideal solvent. This reaction is a reversible reaction. In order to make the reaction proceed to the right, an excess of SO2 is added to the reaction system, and anhydrous methanol can dissolve a large amount of SO2. In addition, anhydrous methanol acts as a solvent to prevent side reactions, so anhydrous methanol is used. Become the solvent of choice.
2 blank test
Usually 20 ml of anhydrous methanol (content: 99.5% or more, analytically pure) is added to the reaction cup (5 hours drying in an oven at 50 ° C), just submerging the double platinum electrode, and then adding Karl Fischer reagent to the burette (single component safety) Type pyridine-free Karl Fischer reagent), turn on the electromagnetic stirrer (60 rev / min), start titrating the water content in the methanol, slowly titrate to the instrument screen display blank finish, do not record the volume consumed by Karl Fischer.
3 calibration
In order to investigate the stability and accuracy of the entire operating system, we should calibrate the system before the sample is tested (including instrument calibration and Karl Fischer reagent calibration). The Karl Fischer moisture analyzer usually uses a methanol-water standard solution, aqueous sodium tartrate, distilled water, saturated water toluene, etc. as a standard to verify the reliability of the method. Aqueous sodium tartrate (C4H4NaO6·2H2O) is a commonly used aqueous standard material. Under normal conditions, the substance contains 15.66% water, which is stable and does not absorb water or water. The material has a weight loss of 15.65±0.02% at 105 ° C and long-term exposure to air with a humidity of 20 to 70% in increments of 0.01 to 0.09%. The author recommends that when measuring trace water (1% or less), use aqueous sodium tartrate as a reference to calibrate; when measuring constant water (not less than 1%), use distilled water (or deionized water) as a reference to calibrate. The Chinese Pharmacopoeia (2005 edition) standard stipulates that the calibration test should be performed three times with an error range of ±1%.
4 sampling, determination
Weigh accurately and mix the appropriate amount of the test sample (about 1 to 5 ml of Karl Fischer reagent) and measure it directly with a Karl Fischer moisture analyzer. The specific operations are as follows:
1 Put the sample into the weighing boat in the appropriate amount, put it into the electronic balance, press the “tart†key to return to zero;
2 Pour the sample into the reaction vessel, taking care not to sprinkle the sample onto the electrode or the wall of the cup;
3 Put the weighing boat on the electronic balance again, and determine the weight of the added sample by weight loss method;
4 Enter the sample weight data (accurate to 0.0001g) into the instrument and wait for the reaction to end until the instrument screen displays the titration result;
5 After the end of the experiment, rinse the instrument line with anhydrous methanol, because the crystals precipitated by Karl Fischer reagent will cause tube blockage.
Five considerations
In addition to determining the nature of the drug, the method of determination, the selection of the calibration material, the sampling method and the size of the injection affect the accuracy of the measurement, the following three issues must be noted to ensure the accuracy of the measurement.
1 Installation and location of the instrument
1 The instrument shall not be installed in a room with corrosive gas, because corrosion of corrosive gas to some parts of the instrument will seriously shorten the life of the instrument;
2 Do not install in an unstable power supply. It is recommended to use an electronic voltage regulator.
3 Avoid direct sunlight to the instrument;
4 The room temperature of the instrument installation site should be controlled within the range of 10 ~ 35 ° C; the relative humidity should not be greater than 60%.
2 double platinum electrode maintenance
When the measurement double platinum electrode is contaminated, it should be cleaned, otherwise the sensitivity of the electrode will be lowered, which will affect the accuracy of the measured data. There are three commonly used electrode cleaning methods: 1 can be wiped clean with acetone or absolute ethanol; 2 the electrode is placed in distilled water for ultrasonic cleaning; 3 if the above two methods can not remove the dirt, the electrode can be in the chromic acid washing solution Soak for two minutes, then rinse off with distilled water.
3 Sealing and protection of the instrument system
In the Karl Fischer's titration process, the wrong end point (ie, reaching the end point in advance) sometimes occurs, resulting in a low measurement result. In particular, when measuring a drug having a water content of 1% or less, the effect is even greater, and even measurement cannot be performed. This is mainly because the oxygen in the air oxidizes the iodide ions in the titration cell to iodine, thereby reducing the consumption of reagents. At the same time, the sunlight will also obviously promote the oxidation reaction of oxygen and iodide ions, so it is necessary to take measures to avoid light. In addition, the Karl Fischer reagent absorbs moisture easily, so a better sealing system is required for the burette and the titration cell (measuring cell) of the titrant delivery system. Otherwise, the end point is unstable and severely affected due to hygroscopicity.
A medium-sized cob of corn provides more than 10% of our daily dietary fibre requirements.
There are two types of dietary fibre - soluble and insoluble - and sweet corn contains both.
According to the American Heart Association, dietary fibre as part of an overall healthy diet can help lower blood cholesterol levels and may reduce the risk of heart disease. It is insoluble fibre that binds to cholesterol, preventing it from being absorbed into the bloodstream.
Insoluble fibre is responsible for promoting regularity and helping to prevent constipation by speeding up the passage of food and waste through the intestines and absorbing water to keep stools soft. Insoluble fibre has been shown to reduce the risk of haemorrhoids.
Fibre-containing foods such as sweetcorn also help to provide a sense of satiety and may therefore help to suppress appetite and aid weight management.
Dietary fibre has also been linked to a reduced risk of type 2 diabetes. A diet rich in fibre helps patients manage their disease.
Fibre is fermented by bacteria in the colon. Promising studies are underway to determine the health-promoting effects of fibre fermentation breakdown products, for example, short-chain fatty acids, which may help to maintain a healthy gut.

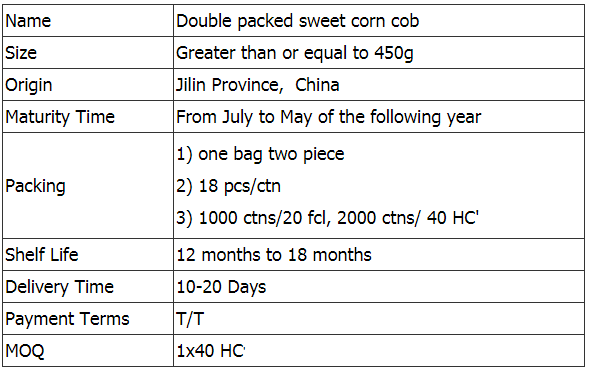
Yellow Sweet Corn,Double Packed Sweet Corn,Double Packed Sweet Corn Cob,Double Packed Yellow Sweet Corn
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nongsaocorn.com