Thoughts on the development of biomedical innovation - from scientific research to industrialization: the last 5 kilometers of the development of biomedical industrialization innovation!
(Author: High St. biomedical Yong)
Zhou Qi, a researcher at the Institute of Zoology of the Chinese Academy of Sciences and a member of the Chinese Academy of Sciences, said: "Real scientific research and innovation should start with science and technology planning." If the scientific research plan itself is traceable and imitative, there is no major innovation under such a framework. Only farther and farther.
Since 2003, China's investment in basic science has increased year by year. Many universities and research institutes have been strengthening their talents and equipment, and projects to high-level academic achievements. The number of SCI publications has also increased from rare to second in the world. The investment in research and development funding is also in the top three in Asia. However, the ability to truly transform scientific research results and innovative achievements into products is still far from the developed countries in the world;
The first part: the status quo of China's biomedical innovation development:
At present, China's basic scientific research is undergoing an important transformation from tracking to parallel development. However, there are also some problems, such as the lack of original achievements in the frontiers of development, insufficient talents for science and technology, unbalanced academic layout, weak cultural atmosphere, and the basic mechanism to support and lead the development of the industry is not mature enough.
The current team of researchers is very large and has made significant progress in many areas of technology. However, the conversion rate of China's scientific and technological achievements is only 10%. There is still a great distance compared with developed countries;
At present, the world scientific research pattern is undergoing an important transformation. From 2001 to 2011, global R&D expenditures grew at an average annual rate of 6.7%. In 2011, global R&D expenditures exceeded $1,435 billion (in 2005, 105.1 billion, and in 2001, 753 billion).
The top seven countries that invested the most in 2011 accounted for about three-quarters of the world, and the top three countries in the United States, China, and Japan exceeded the global 50%. The United States remains the largest R&D executor. In 2011, its total R&D expenditure was $429 billion, accounting for almost a third of the world's total, but its share fell from 37% in 2001 to 30%.
The innovative achievements of scientists in colleges and universities can't find a direction, the way out, medical entrepreneurs can't find good innovation projects; national repetitive research, repetitive projects have increased, and various forms have been formed in the industrial chain of biomedical industrialization. Information islands; social resources integration, the combination of the upper, middle and lower reaches, the combination of technology and capital, the combination of domestic and foreign countries, the establishment of a channel for information flow; the formation of tradable technology trading centers and markets is very necessary of.
"Innovation, cooperation, development, and win-win"! Open the last 5 kilometers of biomedical industrialization innovation development! It is the direction of our determined efforts!
The second part: China's biomedical innovation development thinking:
The development of biomedical innovation should:
The connection between scientific and technological achievements and pharmaceutical companies is a process from “0†to “10â€, and pharmaceutical companies can bring the results of “10†to “infinity†to the market! Therefore, strengthening the innovation of biomedical sources and industrialization is a problem that every medical person should think about.
The development of biomedicine from laboratory research innovation to industrialization is a rather complicated and lengthy process. Only from the source of innovation, intellectual property protection, platform incubation, capital promotion, clear positioning, resource integration, and determination will be able to bear fruit!
“Open cooperationâ€, “targetingâ€, “source innovationâ€, “international visionâ€, “clear positioningâ€, “resource integrationâ€, “intellectual property protectionâ€, “business model innovation designâ€; “industry capital marriageâ€, "Lab, incubator base, industrial park triple jump!".
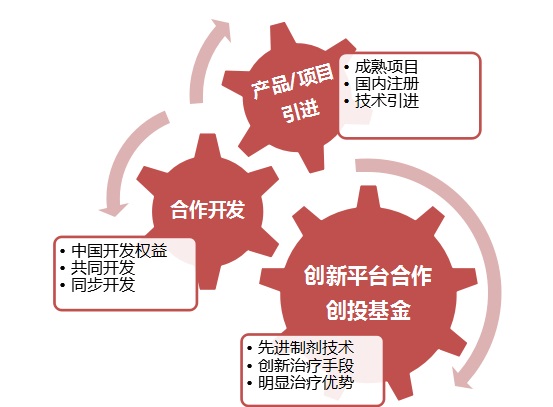
The figure below shows:

Many studies in China are still in the preclinical stage, and in the laboratory stage!
Trend 1. Introduce mature technologies and products through in license.
Trend 2, Co-development has become the mainstream.
Trend 3, innovation platform, and the integration of venture capital funds have become the new darling of future cooperation.
1. Biomedical innovation development should focus on open cooperation
1. Open cooperation means having an open mind and a cooperative attitude; on the road of innovation, we should listen to the experience of experts, professors and different teams in the pharmaceutical industry; cooperation and division of labor can be carried out in the field of cooperation; For example, in the development of genetic drugs, you can choose a platform that can be trusted upstream for plasmid construction, target screening, intermediate intellectual property rights can choose the platform for declaration, downstream production, industrialization can choose platform for cooperation.
2. Open cooperation can give full play to the advantages of each team. Joint development, 1+1 is greater than 2 effect.
3. Open cooperation can stimulate the dream of researchers, and it can also let pharmaceutical commercialization companies do physical business and see the direction.
4. Open cooperation can look beyond the eyes, open your heart and cooperate with a win-win mentality.
Second, the development of biomedical innovation should pay attention to the international perspective
This is very important for companies that are making biomedical innovations. In the past 20 years, China has mainly relied on chemical drugs, mainly Chinese medicines; the proportion of biological drugs in China is quite small.
1. Keep up with the research and development direction of international pharmaceutical companies. Vigorously develop bio-pharmaceuticals and innovative drugs.
With the increasing cost and difficulty of traditional new drug development, the development of human DNA recombination technology, genetic engineering, protein engineering and "group", biopharmaceuticals are becoming a new star in the pharmaceutical field, bringing the gospel to the majority of patients. Seven of the top 10 global sales in 2014 were biopharmaceuticals, five of which were monoclonal antibodies.
For the Chinese bio-product market, autoimmune diseases such as tumors, organ transplant rejection, and rheumatoid arthritis are the three most imaginative areas. The market space for monoclonal antibodies is expected to be around 25 billion yuan. According to statistics, from 2001 to 2015, the investment in China's monoclonal antibody research has increased year by year, and the number of declarations has increased significantly since 2010, reaching a record 41 in 2015.
2. Compound innovation has become a new trend in the international and domestic.
New drugs approved by the US FDA are now more and more focused on biotechnology products, and about half of the new drugs approved last year are biotech drugs. It has now been developed to antibody plus antibody, bifunctional antibody;
The drug can be combined with the gene, and personalized drug delivery developed by high-throughput sequencing.
Innovations in pharmaceutical dosage forms, as well as identification of children's medication packaging, ease of use and above.
3. Select international regions to conduct research on new indications.
In biomedical innovation, we can choose new, well-tolerated indications that are not currently on the market. We can also use new indications for existing drugs, new indications without listed drugs, or existing drug candidates. New indications can also be listed in developing countries or new indications in different regions.
4. Choose a good international two-way cooperation mechanism.
In biomedical innovation, we can make full use of international R&D resources, excellent projects, technologies, and talents can be introduced to the country; domestic good projects, technology can also find good foreign investors.
Third, biomedical innovation development should focus on resource integration
1. Whole, learn, borrow, use; we can use in the development of biomedical innovation.
2. The development of biomedical innovation should give full play to the cooperation of professionals in R&D, technology, talent, transformation, patent, capital, management, park, government, registration, investment promotion, planning and sales in the biomedical innovation industry chain.
3. Fully complete SWOT analysis of your own team and your own projects before integrating resources.
Analyze the advantages of the team, what position it is in the entire industry chain; analyze its own deficiencies; analyze the factors that the external environment is beneficial to our team; the external environment is unfavorable to us; what is missing and what to add.
4. Design and standardize the industrial map before integrating resources.
The focus of the smile curve is to capture: brand, core technology, talent and sales channels;
In the process of the development of our bio-pharmaceutical industry, it is not necessary for the self-employed team to personally cooperate; cooperation can save time, save costs and achieve a win-win situation.
(1) The development of biomedical innovation should focus on cooperation with well-known associations at home and abroad.
(2) The development of biomedical innovation should focus on cooperation with well-known university research institutes.
(3) Biomedical innovation development should focus on cooperation with well-known conversion platforms.
(4) Biomedical innovation development should focus on cooperation with the government and the park.
(5) Biomedical innovation development should focus on cooperation with high-end talent headhunting companies and various elite clubs.
Fourth, biomedical innovation development should focus on strategic intellectual property protection
1. Play an important role in the development of intellectual property in innovation-driven development;
Innovative development of biomedicine, intellectual property first; relying on the strength of team research, create more high-value patents, and promote the transformation of intellectual property from superior to superior and from large to strong.
2. Establish a corporate intellectual property group, the general manager is the team leader;
Enterprises should attach great importance to playing a greater role in the intellectual property talent team and innovative development research.
(1) Have international intellectual property protection awareness and actions.
(2) Intellectual property protection is not just a patent.
(3) Let our intellectual property rights give us real protection and form huge intangible assets and value.
5. Biomedical innovation development should focus on source innovation capabilities;
1. There is no innovation designed from the source just like flying a kite under the table.
Real scientific research and innovation should start with science and technology planning. If the scientific research plan itself is traceable and imitative, there can be no major innovations under such a framework, and it can only be further and further away.
2. Don't innovate for innovation and research and development for research and development.
There is a long way to go for purely technological innovation and transformation; we must form technological protection from technological innovation; but we must also see the feasibility of technology to industrialization;
In addition to technical analysis, we should also analyze our cost advantage from the market, whether our products are in homogenization competition; if we are in homogenization competition, our products still have the value of development.
(1) Innovation to solve problems is a useful innovation;
(2) The innovation that solves the pain points of the industry is the innovation of real industrial development;
(3) The application of new technologies obtained through multidisciplinary intersections is also a new innovation;
(4) Pharmaceutical equipment, drug delivery devices, and auxiliary materials, the production process is a new opportunity for innovation at the source
The development of new excipients is the basis and key to the success of new formulations. "Preparation development, accessories first."
The development of new preparations is in line with the needs and actual conditions of China's development: relatively low investment, short time, high success rate, high added value and more environmental protection. Moreover, the new drug delivery system (DDS) can also produce the role of blockbuster: it has important clinical value, and the market demand is very large. For example, at present, many new drug release systems in China have sold over 1 billion yuan a year, for Chinese medicine. The contribution of the market is huge.
1. Chemical innovation: starting with quality and regulation;
The pioneering (pursuit of goals), tracking innovation (advocating breakthrough), imitating innovation (strengthening follow-up), and imitation (improving connotation) are all advocated for a variety of new drug research and development models.
2. Biopharmaceutical innovation: focus on the development of new technologies, the discovery of new protein/gene targets;
The activity, stability and safety of biopharmaceuticals are important.
3. Innovation of traditional Chinese medicine: It can be started from the process and dosage form;
The reform of traditional Chinese medicine preparations must be based on clinical needs, and cannot be changed for "reform", but must be changed for "excellent".
It can also be carried out around clinical positioning, monitoring of adverse reactions, and secondary development of listed varieties.
Sixth, biomedical innovation development should focus on the source
1. Strategic positioning Enterprises that do innovative research and development must have good psychological preparations.
2. The goal must be trade-offs, and the goal must be adhered to.
3. Have long-term goals, medium-term goals, and short-term goals.
7. Biomedical innovation and development should pay attention to clear positioning
1. In our biomedical innovation, our team, the company's energy is limited; must be positioned.
2. With precise positioning, we will make our innovation and research and development goals clearer.
3. The energy and information carried by the corporate brand is limited, and it is necessary to learn to divert.
Eight, biomedical innovation development should pay attention to "business model innovation design"
1. The business model is simply a profit model.
2. The business model also includes the team and the stable development model of the company.
3. The equity distribution model and the equityization of the biomedical innovation technology team are very important.
Nine, biomedical innovation development should pay attention to industrial capital marriage
1. The development of biomedical innovation is a very important part of capital.
2. PE/VC only makes short-term investments; and the long-term nature of our biomedical innovation development is not feasible.
3. The reason for the failure of most asset restructurings is because the two teams are culturally incompatible; the objectives are inconsistent.
4. Biomedical innovation development should focus on industrial capital marriage to improve the success rate and survival rate of the project.
X. The development of biomedical innovation should pay attention to: “Laboratory innovation, incubator base assistance, industrial parks settled in triple jump!â€
1. The biomedical industry should help develop the design to be reasonable.
2. Universities can set up small innovative transformation centers; a city has multiple biomedical incubation bases to provide convenience for entrepreneurial teams; the park's preferential policies should be more, more in place, and more widely publicized.
3. To open up a new model of cooperation between university researchers and enterprises, such as scholars to establish laboratories in enterprises, or enterprises to set up laboratories in colleges and universities to promote the high degree of integration of production, education and research.
Of course, the development of biomedicine innovation must establish a sound financing system, establish an incentive mechanism for talent introduction, deepen institutional reform, and create a good policy environment. With our open mind, positive attitude, accurate intelligence, and good cooperation, we can all Get a win-win development.
(Thank you all, the above only represents my own point of view, welcome everyone to exchange and criticize and correct!)
About Chongqing Gaosheng Biomedical Co., Ltd.
Chongqing Gaosheng Bio-Pharmaceutical Co., Ltd. is a global comprehensive drug research and development, biomedical innovation achievement transformation service company. The headquarters is located in the High-tech Pioneer Park of Jiulongpo District, Chongqing. Biomedical industry incubator, scientific research technology transfer, scientific research achievements transformation, preclinical experiments, clinical experiments, in vitro molecular diagnostics, graphene nano-new materials research in one biomedical high-tech company, "China Pharmaceutical Enterprise Development Promotion Association" Board of Directors unit. The company is committed to promoting the corporate culture of truth, kindness, trust and harmony, and adheres to the concept of technological innovation and the rejuvenation of the country through science and technology. Gaosheng Pharmaceutical Whole Industrial Chain Transformation Center has many years of experience in bio-pharmaceutical industry chain transformation, and can realize one-stop service from new drug research and development, technical support, fund docking, drug approval, admission and product listing. . Years of new drug development and industrial transformation experience have enabled Gaosheng Pharmaceutical to have a large amount of practical experience, rich industry resources, and a unique sense of innovation.
About Gao Sheng Biomedical Zhou Yong
Zhou Yong: Founder of Gao Chuanghui International Innovative Drug Incubation Platform, Gao Sheng Pharmaceutical Technology Co., Ltd.: President, Executive Director; Medical Laboratory and Business Administration, Chongqing University EMBA, Tsinghua University CMO, Peking University School of Medicine Post-EMBA; Pharmacy, basic scientific research, molecular biology, cell biology, pathology, proteomics, production management, sales management, multidisciplinary background in personnel management and rich experience. Engaged in biomedical scientific research management and innovation and transformation for ten years, and has a unique insight into the innovation and transformation of biomedical industrialization.
Good at: corporate strategic planning, integrated marketing, cutting marketing, team building, corporate culture construction, bio-industry industry transformation and innovation control, brand building, platform construction, project docking, biomedical achievements transformation.
He has worked in: TAKARA, Sino Pharmaceutical, Southwest Bioengineering Pilot Base and other large pharmaceutical and pharmaceutical industries, engaged in technical, R&D, human resources, marketing planning, brand building and other related senior management;
Sunflower Kernel,Sunflower Seed Kernels,Flavored Sunflower Kernels,Raw Sunflower Kernels
Wuyuan county dafeng oil food co.,ltd , https://www.dafengfood.com.cn