Motorized door pulsation vacuum sterilizer technical file
- product description
Motorized door series pulse vacuum sterilizer is one of the series of high-grade sterilization equipment developed and produced by Xinhua Medical according to the new requirements of the national sterilization equipment. It is a high-grade disinfection and sterilization device that fully meets the requirements of GMP regulations. It is also a hospital and pharmaceutical. An ideal replacement for bioengineering and other industries. This series of sterilizers can be widely used in the fields of bioengineering, medical and health, laboratory animals, pharmaceutical industry and other fields to sterilize biological products, utensils, sterile clothing, medical equipment, medical dressings and other items with extremely high sterilization requirements.
- The sealed door adopts electric lifting and compression gas sealing technology, which achieves reliable sealing and greatly reduces the labor intensity of the operator's opening and closing door, and makes the automation degree of the sterilizer reach a new level.
- The upper computer adopts the touch screen as the man-machine control interface, which can dynamically display the temperature, pressure, time and other parameters in the workflow and working process, making the operation more intuitive and convenient. The user can also perform special configuration and convenient manual according to the needs. operating.
- The lower position machine adopts the modern new control device--programmable controller (referred to as PC) for program control, featuring strong function, high reliability and flexible use.
- The air is removed by mechanical forced pulsating vacuum, and the steam is injected multiple times by multiple vacuums to completely eliminate the cold spot in the sterilization chamber, so that the air removal amount reaches 99% or more, completely eliminating the temperature "dead angle" and "small capacity effect". ", to ensure a reliable sterilization effect.
- The sterilizer is divided into single sputum and double sputum (both single door and double door). The double sputum sterilizer can effectively isolate the bacteria area from the aseptic area to meet the national GMP requirements for pharmaceutical production management.
- The main control parts and valve parts are all selected from international high-quality parts, which greatly improves the stability and reliability of such equipment.
- Model and naming

- Structural features
- Model construction: The sterilizer consists of a main body, a sealing door, a piping system, a control system, and an exterior trim cover, and is equipped with a sterilizing vehicle and a truck.
- The main body is double-sided welded sandwich to strengthen the structure. The inner chamber is made of imported stainless steel plate with excellent corrosion resistance and is automatically welded by special plasma welding machine. The surface is mechanically polished and electrochemically polished, which is smooth, corrosion-resistant and durable. The interlayer is made of Q235-B high-quality carbon steel plate, which is designed and manufactured by strict technical process and testing process. It is a main structure with novel structure and high pressure.
- The sealing door adopts electric lifting, air pressure sealing structure and safety interlocking device. When the door does not enter the main body hole position, the door cannot be raised and lowered, and the program can be started when the door is lowered to the normal sealing position. At the same time, once the program is started and running, the sealed door is locked and cannot be opened to ensure safe operation.
- The pipeline system consists of a control valve, a vacuum pump, a condenser and a pipe. The control valve adopts the imported angle seat type pneumatic valve and the first conductive magnetic valve, and the pneumatic valve has no fault action up to 4 million times. The vacuum pump is directly connected, mechanically sealed, low noise, no water leakage, no vibration.
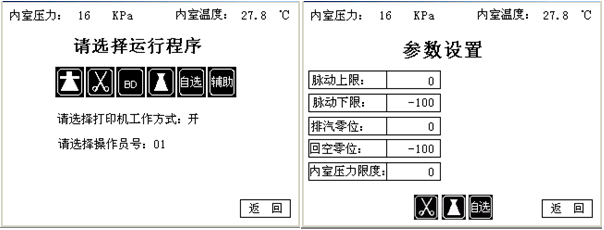
- The control system selects PLC as the main controller, touch screen as the man-machine interface, Japan SMC production pressure controller and other components form a complete control system, generally equipped with five sets of sterilization procedures (fabric program, equipment program, liquid program) , BD experiment, optional program), you can choose the application according to your needs, or you can change the working parameters according to the actual situation. The touch screen displays the status of each process run by the program in real time.
6. The joists, disinfection vehicles and vans configured in this equipment are used for loading and transporting sterilized articles, which are beautiful in appearance, convenient to use and durable. Based on clean and hygienic standards, the sterilization vehicle brackets are all made of stainless steel.
- The exterior decorative mask is an all-stainless steel brushed plate, which is beautiful in appearance and easy to scrub.
Fourth, the sterilization process
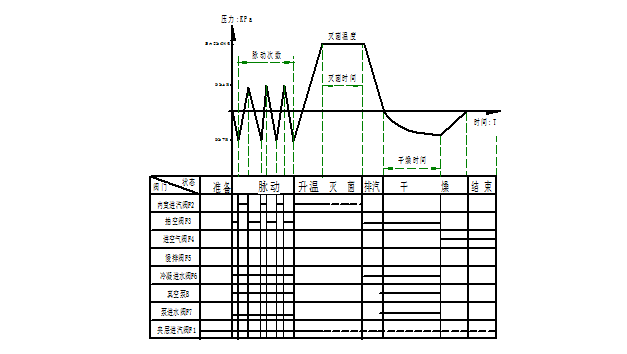
- "Fabric" procedure: Suitable for sterilization of items such as fabrics.
Human and automatic human human automatic button
Open the door - → load the car - → enter the sterilization room - → close the door - → the program starts - →
Automatic pulse count to the lower temperature limit to the sterilization timing to the pressure to zero
Pulsating vacuum - → warming - → sterilization - → exhaust steam - → vacuum drying - →
Dry timed to human pressure to zero automatically
Into the air - → end - → open the door - → unloading
Its working curve is as follows:
 2 "liquid" procedure: only suitable for liquid evacuated or unwanted items.
2 "liquid" procedure: only suitable for liquid evacuated or unwanted items.
Human and automatic human human automatic button
Open the door - → load the car - → enter the sterilization room - → close the door - → the program starts - →
Automatic temperature sterilization to limit the timing to human pressure to zero automatically
Inlet steam rises - → Sterilization - → Slow exhaust - → End - → Open the door - → Unloading
Its working curve is as follows: 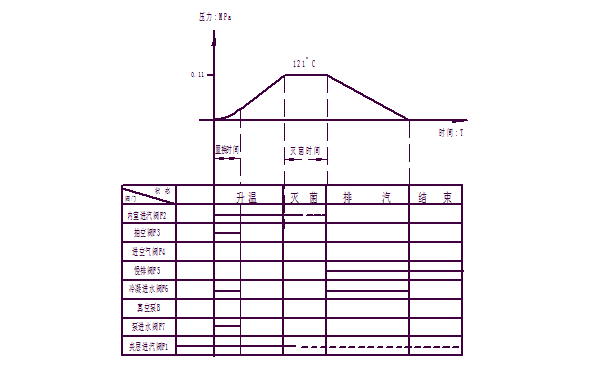
Five, performance characteristics
- The main components of the sterilizer are made of international standard and adopt the world*SUS304 stainless steel material. The parts that are in contact with the sterilized articles are mirror polished, smooth and flat, no dead angle, easy to clean, avoiding the residual bacteria caused by the traditional matte. Breeding, fully comply with US FDA standards and GMP requirements, and also meet European IDF standards. In particular, the main body adopts a new plasma welding process, which greatly improves the internal and external quality of the sterilizer.
- The pulsating vacuum method is used to make the sterilization more complete and thorough; the dressing is vacuum dried to greatly shorten the drying time.
- The touch screen control greatly reduces the labor intensity of the operator, making the entire sterilization monitoring process more intuitive and convenient. The temperature, pressure, time, process stage and preset parameters of the sterilization process are automatically displayed on the touch screen display and can be stored at any time, and equipped with a micro printer for printing process parameters for archiving and future reference. In addition, the control system is equipped with a complete self-test and calibration program, which can correct the parameters such as pressure and temperature at different altitudes and different regions; it also has multi-level control protection and help functions.
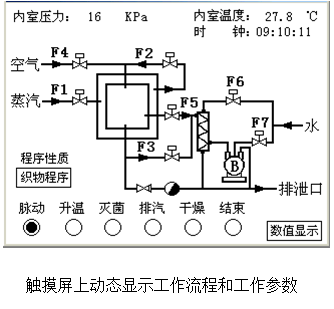
Dynamic display of workflow and operating parameters on the touch screen
- The sealed door of the sterilizer is designed as a motorized door that slides up and down. Double-door type to prevent cross-contamination, front and rear door interlock control in electrical control, separate different levels according to the standard. The compressed air seal, the door is not closed, and the program cannot be started. When there is pressure in the sterilization chamber, the sealed door cannot be opened to ensure equipment and personal safety.
- Among the domestic similar products, Zui has adopted the angle seat pneumatic valve to ensure 4 million trouble-free operation.
- A special air filter with a filtration accuracy of 0.2 μm is selected to ensure no secondary pollution after sterilization.
- The main components of the sterilizer are insulated by high-quality insulation materials, so that the thermal auxiliary injection is reduced to a low limit and the working environment is protected.
- The residual steam in the interlayer and the inner chamber is discharged in the form of condensed water, and does not pollute the ring mirror.
6. Description of special technical requirements:
- If there are special requirements for the inner casing and jacket material, it should be specified in the contract.
- If the inner chamber and the mezzanine are separately fed into the steam (that is, the two-way steam is used), it should be specified in the contract.
- If the disinfection vehicle needs to be adjusted, it should be specified in the contract.
- If there is any difference, the main component configuration table in the formal contract shall prevail.
- If there are other requirements, it should be specified in the contract.
Palmitoyl Pentapeptide-4,argireline,GHK-cu,Acetyl,Hexapeptide-8
PYSON Co. ,Ltd. , https://www.pysonbio.com