The medical device industry is generally regarded as a sub-sector of the pharmaceutical industry. The relevant policies of the pharmaceutical industry are bound to affect the medical device industry. Today, the Chinese pharmaceutical industry is in the process of accelerating the integration of high-quality resources and the elimination of backward production capacity.
According to the sample survey of the China Medical Materials Association Medical Devices Branch, the sales volume of China's medical device market in 2016 was about 370 billion yuan, an increase of 62 billion yuan over 2015, with a growth rate of 20.1%. The overall market size of medical devices continues to expand, but the medical device circulation industry still has a widespread phenomenon of “scatter, miscellaneous, and inefficientâ€.

According to the 2016 Annual Report on Food and Drug Regulation Statistics released by the CFDA, as of the end of November 2016, there were 15,343 medical device manufacturers in the country, including 4,979 enterprises that can produce one type of products, and 8,957 enterprises that can produce second-class products. Home, 2,366 companies that can produce three types of products. Compared with 2015, 1868 medical device manufacturers have (planned) cancellation of the "Medical Device Manufacturing Enterprise License". In 2016, the relevant policies implemented by the state have a great impact on the medical device industry. The most influential one is the “two-vote systemâ€.
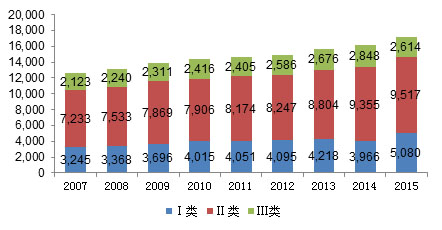
Chart 1: Changes in China's medical device manufacturing enterprises from 2007 to 2015 (unit: home)
The products of the medical device industry are mainly divided into four categories: low-value consumables (common supplies for nursing departments represented by indwelling needles, infusion sets, etc.), high-value consumables (mainly for cardiac intervention and orthopedic intervention), in vitro diagnosis (testing) Equipment + reagents) and large equipment (based on diagnostic imaging and large monitors).
What is the "two-vote system"
The "two-vote system" refers to a drug distribution system in which a pharmaceutical production enterprise opens a ticket to a pharmaceutical circulation enterprise, a pharmaceutical circulation enterprise opens a ticket to a medical institution, and compresses the circulation link with the "two votes" as a standard to improve circulation efficiency.
The "two-vote system" is an important policy implemented by China in the circulation of medicines. It is an important reform measure. The purpose is to reduce the circulation of medicines, make the price of circulation links transparent, further reduce the price of medicines, and reduce the burden of drug use.
Organic fructose syrup is also called high fructose syrup or isomerized syrup. It uses refined corn starch as raw material. The saccharification liquid obtained by enzymatic saccharification of starch is subjected to the action of glucose isomerase to convert part of the glucose into fructose, which contains glucose. A mixed syrup with fructose. Fructose syrup is a starch sugar with high sweetness. It is colorless and odorless, has a pure sweet taste, and has good fluidity at room temperature. It is a new type of sugar source to replace sucrose.
Fructose Syrup,Organic Fructose Syrup,Corn Fructose Powder,Organic Fructose Powder
Organicway (xi'an) Food Ingredients Inc. , https://www.organicwayince.com