Recently, Grail is preparing to invest $1 billion to recruit 7,000 newly diagnosed cancers and 3,000 healthy people, and is committed to the development of early diagnostic markers for cancer blood tests. Is the tumor blood test going to break out?
The progress and prospects of tumor fluid biopsy are spit out from the perspective of technology laymen and users. Throwing bricks to attract jade.
Comparison of advantages and disadvantages between tumor liquid biopsy and traditional tissue biopsy
When attending the cancer liquid biopsy conference earlier this year, he still felt that it was far from practical application, and the FDA's guidelines were also vague. More than a year later, a number of liquid biopsy-related diagnostic products have been approved by the FDA, or have received a lot of clinical data, ready to review. The development of liquid (serum) biopsy can be described as changing with each passing day.

Liquid biopsy mainly includes circulating tumor cells (CTCs), cell-free DNAs (cfDNA), exosomes, and the like in the blood. CTCs have been on fire for several years, and after reaching their peak in 2013, they found that the application has many limitations and was replaced by the rapidly emerging cfDNA. Exosomes are still relatively new, although they are developing rapidly, but there is still a distance from the application. The main discussion here is the progress of cfDNA, mainly in the application of diagnostics and real-time tracking of drug efficacy.
The advantages of blood test and tissue test are: convenient, fast, instant, cheap, minimally invasive.
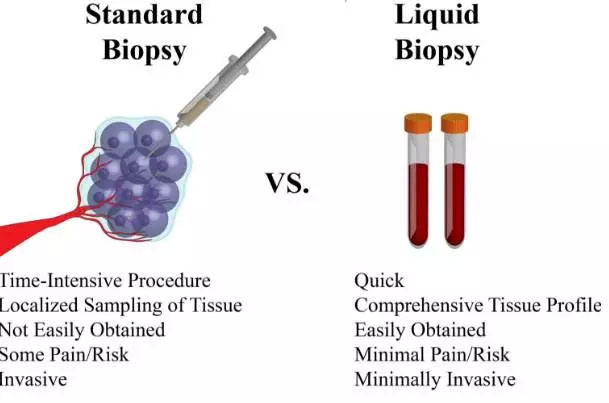
More importantly, blood tests can save many tissue samples that are difficult to obtain (whhen tissue is an issue), or that patients with tissue samples, low quality get targeted therapy, or participate in clinical research. For example, Guardant Health, which completed more than 30,000 clinical tests, reported that blood tests saved up to 50.2% of clinically targeted mutations in clinical patients. These patients were either difficult to obtain for tissue samples, or the sample quality was not high, no A carcinogenic mutation was detected.
Not all tumor cells release the mutated fragment DNA into the blood, and the blood test rates of different types of tumors are also inconsistent. According to Guardant Health, the highest detection rate for liver cancer is about 92%, 89% for non-small cell lung cancer, and the lowest rate for glioblastoma is only 57%. This is one of the biggest restrictive injuries in blood tests. It is also the basis for the organization of biopsy to always have value (still the gold standard).
China manufacturers ,suppliers of Apis Raw,Naphazoline Powder Benefits,Naphazoline Hcl Powder,Neomycine Pharmaceutical API,
Now we have 3 GMP standard workshop, Meanwhile, the factory is equipped with the researching and quality inspection centre, with strong technology research and development strength. We also have 3 salesdepartments over 30 people and sell our products all over the world.
For customer`s needs, OEM service is also acceptable. If you have a good idea in new product production but lack of laboratory device and human resource, we are glad to solve this problem for you. Sincerely hope to strengthen exchanges and cooperation with friends from both home and abroad.
China manufacturers ,suppliers of Apis Raw,Naphazoline Powder Benefits,Naphazoline Hcl Powder,Neomycine Pharmaceutical API
Xi'an Henrikang Biotech Co.,Ltd , https://www.xianhenrikang.com