Over $360 million in new cooperation! Kineta develops non-opioid painkillers
April 19, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Kineta Chronic Pain, a subsidiary of Kineta, announced that it has entered into an exclusive option and licensing agreement with Genentech, a member of the Roche Group, to develop Kineta's α9/α10 nicotinic acetylcholine receptor (nAChR) antagonists. Used to treat chronic pain.

Currently, the treatment of chronic pain is not effective and may have side effects such as addiction and resistance caused by opioid therapy. In the United States, 259 million opioids are prescribed each year, and about 50 people die each day from prescription opioid overdose. This patient population also has significant medical needs that continue to be unmet.
Kineta is an emerging biotechnology company dedicated to transforming “first-in-class†therapies from discovery to proof of concept. Kineta is developing a range of new drug candidates to address critical unmet patient needs in neuroscience, immunooncology, rare diseases and biodefense. Kineta actively seeks to work with multiple parties to promote its innovative research. The α9/α10 nAChR antagonist for chronic pain was developed by Kineta Chronic Pain, a subsidiary of Kineta.
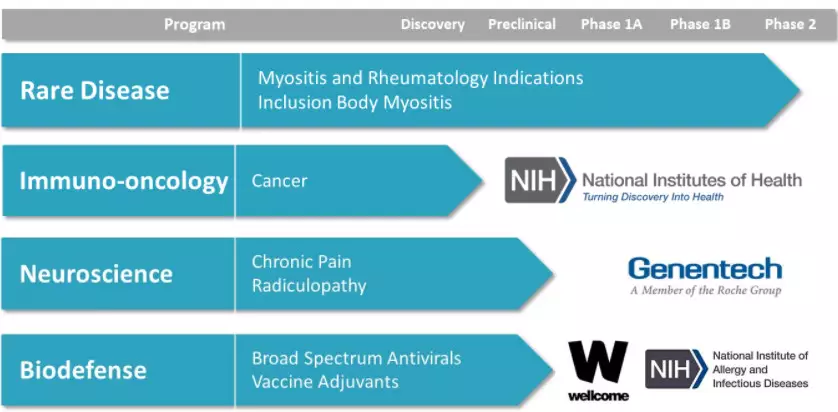
â–²Kineta's research and development pipeline (Source: Kineta official website)
Α9/α10 nAChR is a new target for the treatment of chronic neuropathic pain. Preclinical data show that in-situ compounds that specifically target receptors reduce pain behavior in animal models, exhibiting improved disease effects, including reduced inflammation at the site of injury and neuroprotection. This new target is not expressed in the central nervous system and may lead to safer treatments that are not addictive and non-resistant.
Under the terms of the agreement, Kineta will receive an advance payment and be eligible for up to $359 million in development and promotion milestone payments, depending on whether certain scheduled milestones are met. In addition, Kineta is eligible for the sales royalties for certain products resulting from this partnership. Genentech can choose to license products developed during the partnership. If Genentech exercises its option under the agreement, it will be responsible for the further development and promotion of these products.
“We are very excited to work with Genentech because they are one of the world's leading biotechnology companies and an ideal strategic partner for Kineta,†said Dr. Shawn Iadonato, CEO of Kineta. “Many people with chronic pain need more. Effective, safer and less addictive treatments are a huge unmet need. By working with Genentech, we look forward to accelerating the development of this promising new non-opioid therapy for patients."
“We are excited to work with Kineta to develop potentially life-threatening drugs for patients with chronic pain,†said Dr. James Sabry, senior vice president and global director at Genentech Partnering.
Reference materials:
[1] Kineta to Collaborate on the Development of a Novel Non-Opioid Pain Therapy with Genentech
[2] Kineta Official Website
Medton Medical , https://www.medtonmedical.com