Release date: 2016-03-31
DUBLIN – March 30, 2016 – Medtronic recently announced that the US Food and Drug Administration (FDA) has approved the launch of the Spec® SureScan® MRI surgical electrode with a Medtronic implantable neurostimulator (also known as spinal cord Stimulation, or SCS), for the treatment of chronic pain. In 2013, Medtronic approved the FDA-approved MRI nuclear magnetically compatible implantable neurostimulation system for the treatment of refractory chronic back and/or limb pain, enabling MRI systemic compatibility in specific situations. The approval of the Specify® SureScan MRI® Surgical Electrode made Medtronic the first FDA-approved MRI nuclear magnetic system-compatible SCS system solution. This means that doctors can now tailor a complete set of Medtronic MRI system-compatible SCS systems, regardless of the type of neurostimulator (rechargeable or non-rechargeable) or what type of electrode (percutaneous) Puncture or surgery). The new Specify ® SureScan® MRI electrode will be available this month.
Â
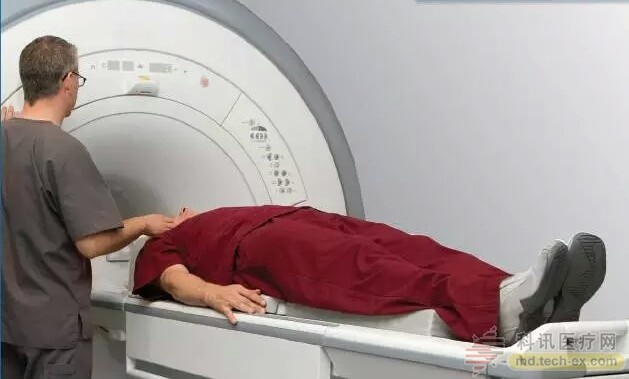
Studies have shown that 82% of patients implanted with spinal cord stimulators require MRI nuclear magnetic examination within five years. The Medtronic SureScan MRI system-compatible neurostimulator system benefits patients and is confident in imaging diagnostics when imaging other diseases is needed. MRI scanning technology has become the standard for disease diagnosis. Through the strong magnetic field and radio frequency pulse signal to form the image data of the internal structure of the body, doctors review the detailed image data to have a more comprehensive diagnosis of the health of the human body, including internal organs. , blood vessels, muscles, joints, tumors, infected areas and other parts of the body. In the United States, the number of MRI scans has nearly doubled in the past 10 years, and the total number of MRIs in 2011 reached 32 million times – more than one per second.
Â
"All patients with spinal stimulators should have the same rights as other patients," said Dr. Steven Falowski, a neurosurgeon at St. Luke's University in Bethlehem, United States. “Unlike the past, patients and medical institutions now care about whether they are compatible with MRI nuclear magnetic scanning when they are involved in implantable devices. This FDA approval means that I can implant my nerve stimulation system into my patients. Treating pain while at the same time giving them the right to receive MRI scan diagnostics in the future."
            Â
Â
It is estimated that 8 out of 10 people suffer from back pain more or less in their lifetime. For some people, non-invasive treatments, such as drugs and physical therapy, are sufficient to relieve symptoms; for others, surgery, nerve block or implanted medical devices are needed, such as spinal cord stimulators or intrathecal Drug infusion pump. Spinal cord stimulation is performed by implanting a subcutaneous pulse transmitter that emits a gentle electrical pulse to the area near the spine. These pulses interrupt the pain signals that are free of the spinal cord and brain to effectively relieve pain and improve quality of life.
Â
“MRI scan diagnostic technology is growing rapidly. Medtronic is proud to offer MRI nuclear magnetic scan compatible SCS solutions for physicians and patients to ensure that patients can receive systemic MRI scan technology without worry, so that patients can receive treatment and timely Help,†said Julie Foster, vice president of the Medtronics and general manager of the pain therapy business at Restorative Therapy Business Group. "Medtronic is committed to driving innovation and advancement in spinal cord stimulation therapy and continues to ensure the availability of compatible MRI nuclear magnetic implant implantable devices, including pacemakers, ICDs and deep brain stimulation systems."
Â
Although the efficacy and long-term benefits of spinal cord stimulation therapy have been supported by evidence-based medicine, some patients who previously implanted SCS cannot fully accept MRI nuclear magnetic scans because the electromagnetic fields generated by MRI scans may result in implanted devices. Damage or damage to the patient's body. These patients can undergo CT scans (computed tomography), but this technique is effective for imaging bone and other hard tissue imaging and does not work well for soft tissue. In some cases, when the patient does need an MRI scan, the implanted device needs to be removed beforehand.
Â
Other effects of Medtronic spinal cord stimulation therapy include:
· Clinical evidence support therapy can significantly relieve pain over a long period of time, helping patients regain their confidence and return to their daily lives.
· Patients can experience an external temporary stimulator for up to 3 to 10 days before the stimulator is officially implanted to assess whether the therapy actually relieves their pain in their daily lives.
· The RestoreSensor® SureScan MRI system is equipped with Medtronic's unique AdaptiveStim® body position control technology, which automatically and automatically adjusts the stimulation parameters as the patient's position changes. The patient does not have to manually adjust the program to accommodate different posture changes.
· Personalized stimulation mode ensures that patients can manage their own pain within preset limits.
Â
Â
The Specify®SureScan® MRI Surgical Electrode and MRI Nuclear Magnetic System-Compatible Implantable Neurostimulation System are not currently available in China.
Â
source:
1 Desai MJ, Hargens LM, Breitenfeldt MD, Doth AH, Ryan MP, Gunnarsson C, Safriel Y. The rate of magnetic resonance imaging in patients with spinal cord stimulation. Spine. 2015 May 1;40(9):E531-7.
2 IMV Benchmark Report 2012. IMV Medical Information Division. Des Plaines, Illinois. Page 1.
3 Web site: https:// Accessed: February 10, 2016.
4 Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomized controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179-188.
5 Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008 ;63(4): 762-770.
Source: Kexun Medical Network
Gws Pumpkin Seeds,Roasting Pumpkin Seeds,Pumpkin Seeds Nutrition,Pumpkin Seeds Wholesale
Inner Mongolia Hengxintonghui Supply Chain Management Services Co.,LTD , https://www.hxthfood.com