Federal regulators have approved the first category of “artificial pancreas†devices that can help some people with diabetes manage the disease by constantly monitoring blood sugar and giving insulin as needed.
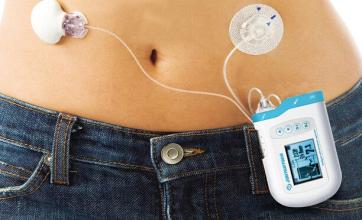
On Wednesday, devices manufactured by Medtronic have been approved for use in patients with type 1 diabetes. Type 1 diabetes is usually diagnosed in childhood. In the United States, with a total population of 29 million, about 5% of this type of diabetes.
Doctors say they look forward to long-awaited equipment that can help patients around the clock.
Currently, patients with type 1 diabetes must manage insulin levels in the body through multiple injections or drug pumps. The patient's own pancreas does not produce insulin, which is the hormone needed to convert food into energy. They face the risks of high blood sugar levels, heart disease and many other health problems.
The new MiniMed 670G unit consists of a drug pump, a sensor for measuring blood sugar, and a tube that supplies insulin. The sensor measures blood glucose levels every 5 minutes and injects or prepenes insulin as needed. Patients still need to manually add insulin before meals.
The US Food and Drug Administration said it approved the device based on a three-month study of more than 120 patients. The study did not report major adverse events, such as dangerous hypoglycemia, suggesting that the device is safe for diabetics 14 years of age and older, the regulator said in a statement.
The drug pump is about the size of a deck of cards that can be worn on the waist or carried in a pocket.
Burglar Safe,Wall Safe Security Box,Office Home Big Safes,Electric Fingerprint Burglar Safe
Ningbo Reliance Security Technology CO.,Ltd , https://www.reliancesafes.com