Release date: 2017-01-16
Looking forward to the upcoming new technologies and tools in 2017 is the New Year's practice. In fact, before 2017 has arrived, we have already predicted that 2017 will be an important year. After all, Medtronic released the first hybrid closed-loop system in the spring of 2016, which opened up a new era of artificial pancreas technology. And this is just one of the exciting developments we've been looking forward to this year.
Artificial pancreas system
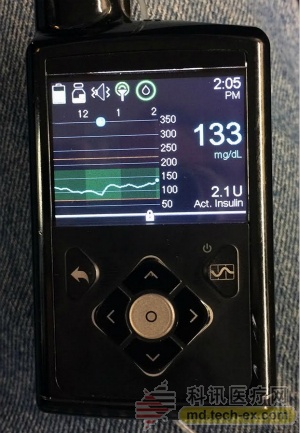
Medtronic's Minimed 670G: In September 2016, the FDA reviewed the Minimed 670G, the world's first system to regulate insulin delivery based on CGM (continuous blood glucose monitoring), which allows users to be as close as possible to their blood glucose goals. The value is 120 mg/dL. The device itself will be equipped with a vertical design layout and a color display. However, the device does not support data sharing. The Minimed 670G features the new Guardian 3CGM sensor, which has improved its accuracy and credibility. The system is directly linked to the Bayer Contour Link 2.4 hand pointer. In 2016, the Ascensia blood glucose meter introduced the Bayer Contour Link 2.4 hand-pointing instrument, allowing the meter to remotely control the use of insulin pills. When talking about the release date of 670G, Medtronic only said "early 2017."
Tandem's first generation of automated insulin delivery devices (AIDs): Manufacturers of insulin pumps plan to release the first generation of closed-loop systems by the end of 2017. The device's characteristic is to predict hypoglycemia pauses (PLGS), which in many ways reflect what the MedT system needs first. The Tandem Diabetes Care Process line shows the current situation and the company's development plans for the next few years.
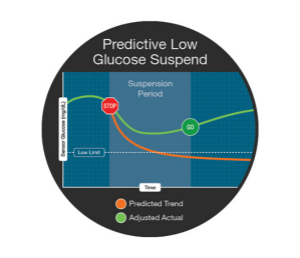
Other artificial pancreatic systems : Yes, we are also observing many other closed-loop systems projects—Bigfoot, iLET, etc.—but we don't expect these to be achieved in 2017, and we need to continue research and experimentation (R&D) .
Medtronic

In addition to closed-loop projects (or can be called cross-projects, depending on who you are asking), Medtronic tells us that they are working on a "holistic" approach to CGM, mobile app and data analysis. In one. Spokesperson Pam Reese said that this is probably the project worth looking forward to in 2017.
Independent CGM: “The development of an independently operated CGM system will be our first step. The independent CGM system can provide insulin injections to users based on real-time data from sensors on the user's smartphone. The system also has text information. The warning feature allows the guardian to receive alerts for high blood sugar levels or low blood sugar levels via the phone. In addition, the system will have the option to automatically upload daily sensor information to CareLink Pro software, allowing health care providers to obtain useful data. Providing care to people with diabetes and adjusting treatments as needed. This will provide clinicians with more point data on trends in blood sugar levels, helping doctors see new conditions and help patients with diabetes optimize treatment method."
Sugar.IQ application: "The first cognitive app we developed in collaboration with IBM Watson Medical is to use this data. This app can help detect important illnesses and blood sugar trends in people with diabetes. Sugar.IQ applications will use IBM Sen analysis methods, analyzing data characteristics and providing real-time, actionable, personalized awareness, so that people with diabetes can enjoy life more freely. This app will provide personal guidance, only through the app platform related diabetes data And observe the management and management of the disease in daily diabetes management."
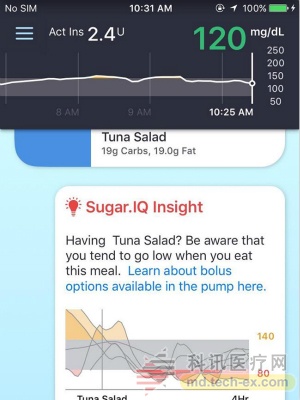
We are not sure of a specific timeline for any new tools, but we hope to hear more about them at the ADA Annual Meeting in June.
Dekang Medical (Dexcom)
Dekang Medical is a leader in innovative products and has been working on a small number of new tools. Although Dekang has submitted three tools to the FDA, we are still looking forward to the fourth tool to be submitted to the FDA in the next few months:
1. Touchscreen Receiver: It is positioned as an upgraded version of the current Dekon Receiver and will be improved for durability and speaker issues.
2. New one-button insertion device: It is expected to be released in the middle of the year. Based on the prototype image we've seen, the device should be similar to Medtronic's Enlite sensor insertion device and can be operated with one hand.
3, small-volume G5 transmitter: will be more compact than the current G6, even smaller than the previous G4 model.
4. New generation G6 sensor: Due to the FDA's exciting dose statement in December, Dekang's plan for a new sensor in 2016 has been delayed, but the plan is expected to begin in early 2017 and may be released at the end of the year. The G6 will be a true leap in CGM technology, extending the current 7-day wear period to 10 days, reducing the number of daily corrections from 2 to 1 and improving accuracy and credibility. This shows the rapid development of the FDA in diabetes technology in recent years, and we are currently optimistic!
Abbott

We hope that Abbott’s novelty FreeStyle Libre Instant Blood Glucose Detector technology will enter the US market in 2017. The system combines fingertip lancing tests with existing CGM (continuous blood glucose monitoring) with a one-point non-invasive technique. In other words, the system consists of a small white prototype sensor on the skin and a "receiver" that looks like a PDM. The user can move the sensor and obtain a glycemic index via a wireless connection.
You can use it often or at your own discretion, as an alternative to saving expensive test paper, and you don't need to run the entire CGM device if you wish.
In early 2016, Abbott Laboratories received a FDA license for the Libre Clinic Professional Edition, and Abbott Laboratories has completed a personal version of the home. Therefore, the issue is only a matter of time. Presumably, patients will be very excited about this!
Johnson & Johnson
Vibe Plus: Vibe Plus is the new name for Johnson & Johnson's next-generation blood glucose pump, because OneTouch removes Animas from its name and seems to be merging all products in the OneTouch brand. In December 2016, the FDA approved the product only six months after the product was submitted.

Several key features: The product still retains 200 insulin agents (consistent with the previous model), waterproof, and is suitable for Glooko-Diasend data sharing software. The product is not equipped with an instrument remote control as well as Ping, and cannot be upgraded at home. But JnJ told us that "remote update capability is a factor in our future product considerations." Vibe Plus has five colors (blue, black, silver, pink, and lime green) and eight personalized skins.
A Johnson & Johnson OneTouch spokesperson told us that they are evaluating the release time and features provided after upgrading the system.

OneTouch Via: Yes, after we introduced OneTouch Via (formerly Calibra Finess) in 2016, OneTouch Via became a wait-and-see project. It is a wearable insulin delivery device that is limited to pills. It has very stable performance and can be worn for up to three days. It has 200 fast-acting insulin agents and, if you press the right button on the patch, allows PWD to consume two units of insulin pills without the need for a separate control unit. In 2010, this patch of blood glucose pump was approved by the FDA for the treatment of T1 and T2 diabetes. In 2012, JnJ OneTouch purchased this technology. But the technology has never been applied in detail and seems to have been forgotten. Until now! If JnJ OneTouch can arrange the release plan for this product, we will look forward to seeing it in 2017.
OmniPod
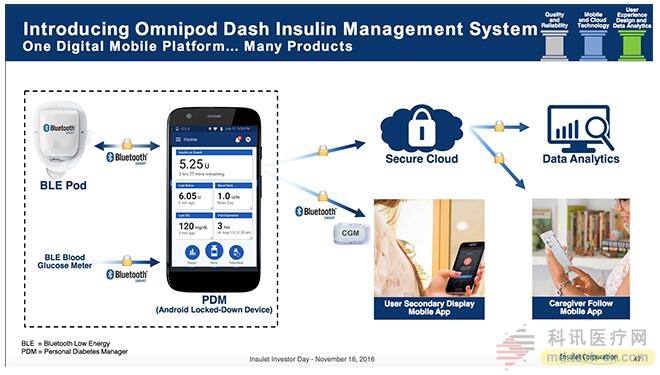
We will look forward to seeing the new OmniPod PDM (called "DASH,") in the ADA Annual Meeting Technology section held in the summer of the 17th. During the "Investor Day" last November, Silver Hugh Company detailed the new Bluetooth control OmniPod plans to debut at the end of this year.
Although this tubeless insulin pump retains the same form factor, it creates a low-power Bluetooth wireless feature that allows connection to the new color touchscreen PDM. This also allows connection to the BT fingertip lancet instrument, however, this means that the next generation OmniPod DASH will be the same as the current insulin pump and will not be equipped with a FreeStyle meter. This is an important drawback for users who prefer integrated devices.
The pleasant thing is that the new BT insulin pump and PDM will be directly connected to the smartphone app. Users can track the insulin content (IOB) on the smartphone display and the new PDM, and get usage records and CGM data. data. To record carbohydrate content, the new PDM is equipped with a comprehensive food database. You can manually enter the BG or use the beautiful touch wheel to choose to enter other data. The new touch screen will be built on the Android system. App data will be locked so it will not be disturbed by other cellular data.
Tandem
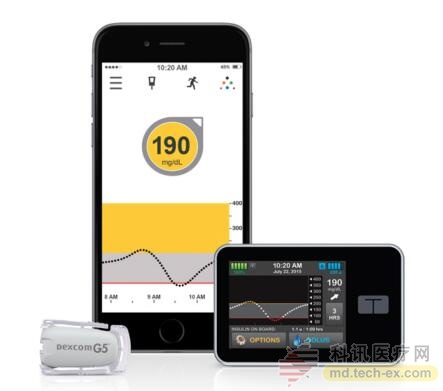
Thanks to the new X2 insulin pump, Tandum is now able to offer remote users the ability to update their devices at home (without having to upgrade or sell like the current D device model). This technology enables the system to be combined with the Dekang G5, which is a project that will be submitted to FDA by Tandem and is expected to be released in mid-2017. Their integrated system also includes predictive hypoglycemia, and existing users can update their devices once they are verified.
Intelligent insulin delivery technology
In the past few months, we have reported two innovations published by Beckton Dickenson:
SMD Pumping for Type 2: This product is completely disposable and can be worn for three days, with both a base dose and a single dose. The details of the current product are not clear, but it is expected to be seen at the 2017 financial conference.
Smart nib technology: This also means that Bluetooth nib technology allows for dose data sharing, as BD is committed to bringing more diabetes interoperability management to its portfolio. The nib technology currently being developed is suitable for all types of insulin pens. about this point--
Campanile Medical Inc. InPen: In July 2016, the FDA approved the product and is ready to appear in 2017. The following points are the product characteristics we mentioned in our first report in July 2015:
Product foundation includes computer chip technology, including Bluetooth LE wireless function
Refillable Lilly or Novo Insulin Pack (the only two available insulin packs in the US)
The pen contains a temperature sensor. If the temperature of the pen drops below the freezing point or rises above the body temperature, the app alarm can be triggered to ensure that the insulin is intact.
Collect user information and transfer it to the app. The app contains a dose calculator that calculates the insulin content (IOB) in real time.
The smartphone app also allows the user to set meal time and snack time reminders, and if you don't take the medication within the set time, the app will issue a warning.
Allow remote monitoring - Users can set the app to automatically send text messages, sending messages to up to 5 people at the same time. The information content includes not only the insulin dose, but also the BG and carbohydrate values ​​(if manually set) - all information will be written in a text message.
At the end of July 2016, the FDA stated that InPen is targeted at patients over the age of 12. Sean Saint, partner and CEO of Campanin Medical, said San Diego plans to release the product on "someday in 2017."
We look forward to a large number of blood glucose meters entering the market in 2017. In particular, two outstanding products from Ascensia (formerly Bayer of Germany) and Swiss Roche Diabetes Medical Company - both have been published in the past few months. We have reported on the Accu-Chek Guide blood glucose meter, which we have predicted sometime this year. Its appearance is very similar to the existing equipment, but it has many cool new features, including no spill test strips. bottle! We are also very happy to hear about the new Bluetooth Ascensia Counter Next One blood glucose meter, which has recently been approved and can be connected to the app to identify symptoms and blood sugar trends. The device is expected to be released in 2017.
Of course, according to the new Dekang drug requirements, fingertips are no longer a necessary step for CGM users, and FreeStyle Libre is about to enter the US market. We have to think about the traditional blood glucose tester market. How long has it been developing? There is no doubt that there will be many things happening in 2017 in research and publicity, such as product channels and financial payment capabilities. We will continue to update the industry information in the circle as always!
Article: healthline.com
Author: Mike Hoskins
Source: Arterial Network
Nucleic Acid Extraction Reagent Kit 96T(auto)
[Intended Use]
Extraction, enrichment, purification of nucleic acid. The processed products can be applied in clinical in vitro detection.
[Testing Principle]
During the nucleic acid extraction process, the magnetic bead adsorption principle is used to adsorb, transfer, and purify nucleic acids through special magnetic beads to automatically complete the nucleic acid extraction.
[Storage and Expiry date]
It could be stored at room temperature.
Expiry date is 12 months.
[Product Features]
1. Efficient extraction of high quality RNA.
2. Rapid purification to obtain high-quality, ready-to-use RNA.
3. Good repeatability and high output.
clinical examination reagent,new coronavirus extraction reagent kit,Covid-19 purification reagent,nucleic acid purification reagent kit,covid extraction reagent kit
Shenzhen Uni-medica Technology Co.,Ltd , https://www.unimedicadevice.com