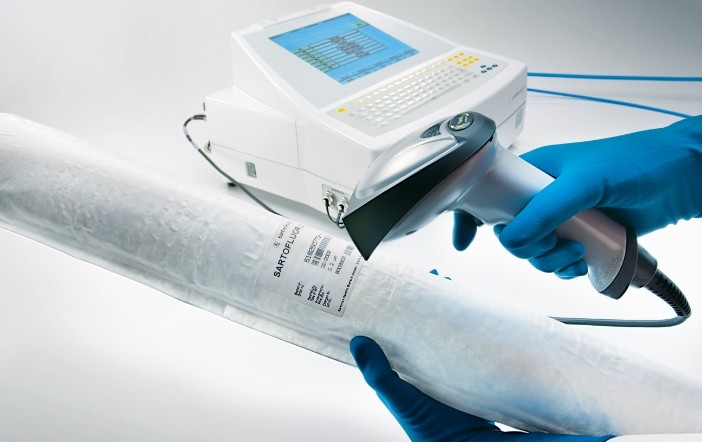
Since the implementation of the new version of GMP, the integrity test of the sterilizing filter has undoubtedly become a key step in the quality assurance of pharmaceutical products for aseptic pharmaceutical manufacturers. In the daily production process, how to eliminate operational errors and effectively improve the success rate of integrity testing, there are three factors worth considering:
1. Both the diffusion flow and the bubble point method are based on the determination of the corresponding integrity values ​​from the upstream to downstream gas flow of the fully wetted membrane. Therefore, when testing the integrity of the sterilizing filter, the wetting filter is the key pre-processing step to ensure the integrity of the integrity test. The process of wetting seems simple, but it is really difficult to achieve complete wetting of the filter. The difference in chemical properties of the membrane material directly determines the choice of wetting method and the ease of wetting. You need to choose the best wetting method for different membrane materials. Considering the simplicity of operation, we usually use the static soaking method, which has no problem for some hydrophilic nylon filter elements, but it will definitely bring about wetting for those modified PVDF membranes. Complete phenomenon. So the best wetting method is dynamic wetting.
2. Is there a better way to do if the optimal dynamic wetting method does not bring the filter element to a fully wet state? The answer is naturally yes. Generally, professional manufacturers will recommend increasing the pressure difference between the upstream and downstream on the basis of dynamic wetting and prolonging the rinsing time. If the effect is not obvious, you can also consider installing a valve at the filter end, and slowly closing the valve as appropriate to keep the whole system in a back pressure state. This can increase the time for the liquid to fully stay, so that the residual bubbles trapped in the folded area can be easily eliminated, thereby achieving the purpose of complete wetting.
3. In addition to the diffusion flow and bubble point methods, there is also a water intrusion method for gas-sterilizing filters for hydrophobic materials, which is also the most difficult to operate in all methods. Factors affecting the stability of the water intrusion method include: water quality, water temperature, ambient temperature change during the test, and the degree of drying of the filter to be tested. The two most important factors are temperature-related. If the water temperature is too low, the flow rate will drop, which will lead to false negative results, which will inevitably lead to the risk of drug production release. If the water temperature is too high, the result will be wrong and will not pass. Therefore, it is generally recommended that the water temperature be strictly controlled at 20-25 °C. In addition, during the test, when the ambient temperature rises, the temperature of the air in the filter housing also rises, and the water temperature remains unchanged, which inevitably leads to a change in the gas compression ratio, thereby affecting the evaporation of water in the system. Changes in traffic cause erroneous results. Therefore, it is best to avoid the occurrence of environmental temperature changes during the test.
The above questions are all about the experience in the actual integrity testing process. Combined with the Sartorius Startocheck 4+ advanced integrity testing equipment, the success rate and accuracy of your filter integrity test will be even more Good protection.
Contact us to discuss issues related to integrity testing with Sartorius technical experts
Plant extracts refer to substances extracted or processed from plants (all or a part of plants) using appropriate solvents or methods, and can be used in the pharmaceutical industry, food industry, daily chemical industry and other industries.

There is a conceptual overlap between plant extracts and herbal extracts. The raw materials of plant extracts in my country are mainly derived from Chinese herbal medicines, so domestic plant extracts can also be called Chinese medicine extracts to some extent.

Herbal Extract,Liquid Herbal Extracts,Herbal Extract Powder,Natural Herbal Extract
XI AN RHINE BIOLOGICAL TECHNOLOGY CO.,LTD , https://www.xianrhinebiotech.com