2014 Innovation Year, China and the United States, the original drug is hot and hot
December 24, 2014 Source: Minenet
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Meters within the network (researcher lily-cha) 2014 Nian may not be a memorable year, but this year a major event occurs, but raise eyebrows. The plunge in global oil prices, the Ukrainian crisis, the continued downturn in the European economy, the decline in the growth rate of emerging economies, and so on, and the negative news of the Ebola outbreak in Africa seem to herald the year. What remains is a sentimental memory. But in fact, this is only one side of things. There are many exciting news in this year. The Indian Mars probe "Mangarian" entered the orbit of Mars. The iPhone 6 with the innovation as the selling point has earned the attention of the world. It also earned huge profits for the company, and Ma Yun became China's richest man in one fell swoop, and also let Ma Yun = "innovation genius" deeply rooted in the hearts of the people.
In the pharmaceutical industry, “innovation, innovation, or innovation†has become the highest slogan of decibels. With Gilead’s innovative hepatitis C drug Sovaldi, it has rapidly climbed to the annual sales of tens of billions of dollars in the subversion of predecessors, and has given new milestones in new drugs. At the same time, innovation became the strongest voice in the medical field in 2014. With the 2014 one hundred nivolumab Myers Squibb fully human IgG4 antibodies are the first to debut in Japan, Merck's KEYTRUDA US FDA approved a new target for therapy molecule programmed death 1 (PD-1) and its ligand against malignant tumor immunity ( PD-L1)'s new drug research and development competition has entered a fever. As the most advanced tumor immunocyte treatment technology, chimeric antigen receptor-T cell (CAR-T) therapy has become one of the hottest areas of drug research and development, and also a hot spot for investment cooperation. CAR-developed by Novartis in July T immunotherapy CTL019 was recognized by the FDA for breakthrough therapy, which also marked the entry of this type of therapy into the harvest season. As the world's most advanced BiTE (bispecific T-cell engager, bispecific T-cell engager) immunotherapy can help the body's own immune system to fight cancer, Amgen BiTE antibody drug Blincyto (blinatumomab) The latest clinical data show After one cycle of Blincyto treatment, 78% of patients experienced complete MRD remission (95% CI: 69-85%), and almost all complete remission (98%) occurred during the first treatment cycle.
Everything that happened in the field of new drug innovation this year was dazzling and overwhelming. Breakthrough, innovation, and exclusive ownership have become the favorite of the major pharmaceutical giants, and have become the trend of major venture capital companies. In order to encourage innovation, the United States has introduced a series of policies, such as the approval of breakthrough drugs, priority approval, rolling applications, patent term compensation, market exclusivity, etc., to protect the innovative enterprises, and major pharmaceutical companies have spared no effort to enter the new drug research and development. In 2014 alone, the FDA approved 29 new molecular entities and 10 innovative biologics.
Table 1 New molecular entities approved by the FDA in 2014
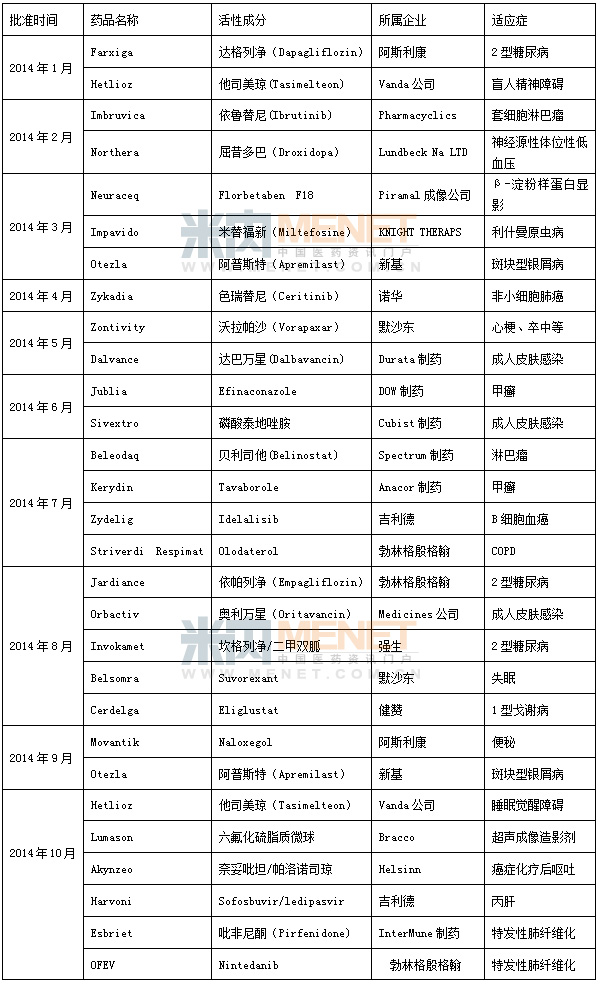
Table 2 FDA approved biological products in 2014
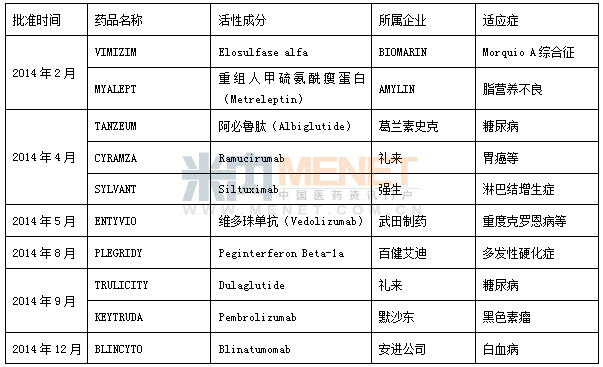
The US FDA approves new drugs based on their chemistry type and therapeutic potential. It is classified into 1 to 10 types according to the chemical type: the first type is the new molecular entity (NME), which refers to the active ingredient that has never been approved or sold as a drug in the United States, and may be a single component or a stereoisomeric mixture. Part of it is calculated as the highest according to the difficulty of research and development. In the classification of chemical drugs in China, a new class of drugs is divided into 6 subcategories, of which 1.1 is also the most difficult category for R&D. With the tilt of domestic research and development of innovative drugs from finance to policy, the company's research and development enthusiasm is also hot. In particular, after a new round of bidding and baptism, the profits of generic drugs produced by many manufacturers have been squeezed to a minimum, and how to break through has become a major issue facing major enterprises. In the face of the eternal theme of "survival or death", how to choose? If you choose to survive, how to survive better, become the main theme of the CEOs of major pharmaceutical companies. Analysis of the intranet drug review database, we found that some companies resolutely chose innovation. The results are gratifying. As a domestic benchmarking enterprise, Jiangsu Hengrui's operating income in the first half of 2014 was 3.51 billion yuan, up 18.23% from 2.969 billion yuan in the same period of the previous year; its net profit in the first half of the year also reached 759 million yuan. Compared with 642 million yuan in the same period of last year, it increased by 18.19%. The company's performance is gratifying, and its R&D investment is also bold. In the first half of 2014, the company invested a total of 264 million yuan, accounting for 8% of its income and 35% of its profits, far higher than the average level of domestic enterprises. As a matter of course, Hengrui has become a domestic first-class pharmaceutical company, and many products have been listed in Europe and the United States.
Table 3 A new class of drugs approved for domestic production in 2014
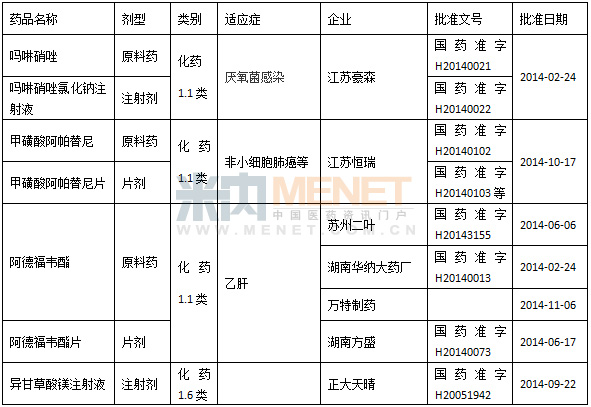
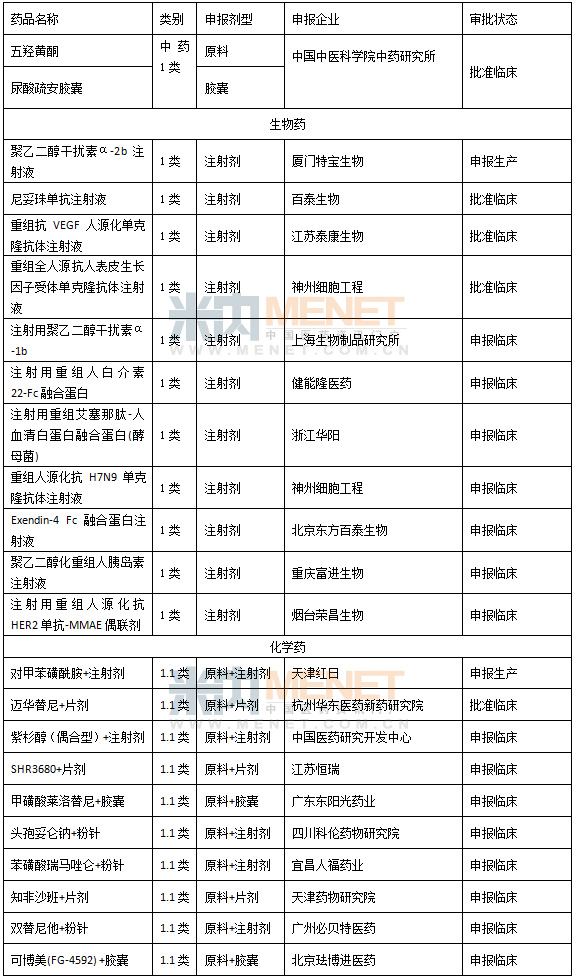
Although the research and development of innovative drugs in China still faces many challenges, with the increase of policy support, the huge opportunities and commercial value in this field also attract many companies to participate in it. According to the data, in 2014, 56 enterprises in China entered the approval of 1.1 new drugs of chemical drugs, and 24 of the biopharmaceutical enterprises also entered the approval of Class 1 new drugs, which were higher than last year. One of the most impressive is the biopharmaceutical products of Shenzhou Cell Engineering Co., Ltd., one of which is currently the most popular all-human anti-tumor monoclonal antibody has been approved clinically, and the other is a special-effect biopharmaceutical against H7N9 avian influenza virus. The sourced anti-H7N9 monoclonal antibody injection" is the world's first special-purpose targeted drug, adding a bright color to this blank field in the world. At present, the product has been applied for clinical.
Table 4 Approval of Class 1 New Drugs in China in 2014 (Chemicals only contain 1.1 classes, and biopharmaceuticals do not contain preventive products)
(Updates closing date all of the above table data are December 22, 2014)
Liu Qianhong, the investment partner of Kaisei, bluntly said: "The era of China's real new drug innovation has begun." This is mainly due to three aspects. First, driven by domestic policies and market demand, enterprises' R&D investment is more daring; second, more experienced medical returnees return to China to start business; third, technological breakthroughs have led to more positive The enthusiasm for new drug investment can be said that a long-term hot spot in the pharmaceutical market is innovation. Are you ready for the wave of innovation in the face of the storm?
Household Cleaner,Grease Cleaner Kitchen,Household Carpet Cleaner,Cleaning Spray
Wuxi Keni Daily Cosmetics Co.,Ltd , https://www.kenicosmetics.com